WARNING LETTER
Humble Juice Co. LLC MARCS-CMS 583547 —
- Delivery Method:
- VIA UPS and Electronic Mail
- Product:
- Tobacco
- Recipient:
-
Recipient NameDaniel Clark
- Humble Juice Co. LLC
9400 Lurline Ave Unit B1
Chatsworth, CA 91311
United States-
- daniel@humblejuiceco.com
- info@humblejuiceco.com
- Issuing Office:
- Center for Tobacco Products
United States
June 7, 2019
WARNING LETTER
Dear Daniel Clark:
The Center for Tobacco Products of the U.S. Food and Drug Administration (FDA) and the U.S. Federal Trade Commission (FTC) recently reviewed the Instagram account of Parker Hornaday (pvrkerr), https://www.instagram.com/pvrkerr, containing social media posts with labeling and/or advertising for several e-liquid products on behalf of Humble Juice Co. LLC, as well as the Instagram account of Humble Juice Co. LLC (https://www.instagram.com/humblejuiceco), the Facebook account for Humble Juice Co. LLC (https://www.facebook.com/humblejuiceco), the Twitter account for Humble Juice Co. LLC (https://twitter.com/HumbleJuiceCo), and the website, https://www.humblejuiceco.com, and determined that the e-liquid products listed there are manufactured, advertised, and offered for sale or distribution to customers in the United States. Under section 201(rr) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. § 321(rr)), as amended by the Family Smoking Prevention and Tobacco Control Act, these products are tobacco products because they are made or derived from tobacco and intended for human consumption. Certain tobacco products, including e-liquids, are subject to FDA jurisdiction under section 901(b) of the FD&C Act (21 U.S.C. § 387a(b)). In addition, the FTC has reviewed the above-referenced social media postings under section 5 of the FTC Act, 15 U.S.C. § 45.
FD&C Act Violation
FDA has determined that several e-liquid products manufactured, advertised, and offered for sale or distribution to customers in the United States by Humble Juice Co. LLC with labeling and/or advertising on behalf of, and by, Humble Juice Co. LLC are misbranded under section 903(a)(7)(B) of the FD&C Act (21 U.S.C. § 387c(a)(7)(B)) and section 903(a)(1) of the FD&C Act (21 U.S.C. § 387c(a)(1)) and/or section 903(a)(7)(A) of the FD&C Act (21 U.S.C. § 387c(a)(7)(A)) because the labeling and/or advertising in social media posts on your behalf, and by you, regarding these e-liquid products fails to include the required nicotine warning statement for these e-liquid products.
E-Liquid Products with Labeling and/or Advertising that Fails to Include the Required Nicotine Warning Statement are Misbranded
Our review of the website, https://www.humblejuiceco.com, revealed that Humble Juice Co. LLC manufactures, advertises, and offers for sale or distribution to customers in the United States e-liquid products, including: HMBL SALT Mango Pineapple, HMBL SALT Blue Blood High, HMBL SALT Strawberry Kiwi, HMBL SALT Apple Jay Jay, HMBL SALT 99 Pink Balloons, and HMBL SALT Watermelon Patch.
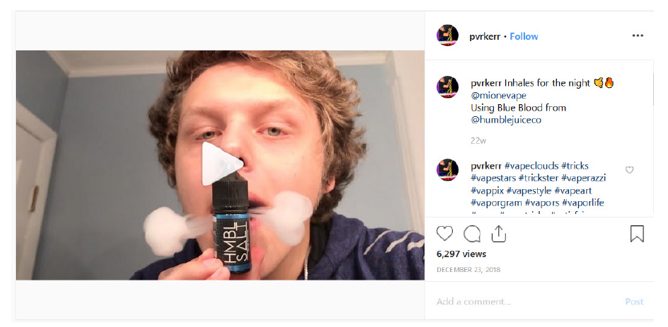
Our review of the Instagram account of Parker Hornaday (pvrkerr), https://www.instagram.com/pvrkerr, revealed that it contains posts on behalf of Humble Juice Co. LLC with labeling and/or advertising for several e-liquid products advertised in the United States that does not include the required nicotine warning statement, for example: HMBL SALT Strawberry Kiwi, HMBL SALT Mango Pineapple, and HMBL SALT Blue Blood High.
Specifically, the social media account of Parker Hornaday (pvrkerr), https://www.instagram.com/pvrkerr, contains posts dated, for example, December 23, 2018, April 28, 2019, and May 22, 2019 with labeling and/or advertising for HMBL SALT Mango Pineapple, HMBL SALT Blue Blood High, and HMBL SALT Strawberry Kiwi e-liquid products that does not include the required nicotine warning statement (see examples below).
Instagram:
Additionally, our review of your Instagram account (https://www.instagram.com/humblejuiceco), Facebook account (https://www.facebook.com/humblejuiceco), and Twitter account (https://twitter.com/HumbleJuiceCo) revealed that they contain posts with labeling and/or advertising for the several e-liquid products advertised in the United States that does not include the required nicotine warning statement, for example: HMBL SALT Apple Jay Jay, HMBL SALT Blue Blood High, HMBL SALT Mango Pineapple, HMBL SALT 99 Pink Balloons, and HMBL SALT Watermelon Patch.
Specifically, your social media accounts on Instagram, Facebook, and Twitter contain posts dated, for example, October 22, 2018, November 8, 2018, November 25, 2018, December 1, 2018, and March 4, 2019 with labeling and/or advertising for HMBL SALT Apple Jay Jay, HMBL SALT Blue Blood High, HMBL SALT Mango Pineapple, HMBL SALT 99 Pink Balloons, and HMBL SALT Watermelon Patch e-liquid products that does not include the required nicotine warning statement.
Under 21 C.F.R. § 1143.3, labeling and advertising for cigarette tobacco, roll-your-own tobacco, and covered tobacco products (other than cigars), such as e-liquid products, must bear the following warning statement:
WARNING: This product contains nicotine. Nicotine is an addictive chemical.
For cigarette tobacco, roll-your-own tobacco, and covered tobacco products other than cigars, it is unlawful for any person to manufacture, package, sell, offer to sell, distribute, or import for sale or distribution in the United States such product unless such tobacco product package bears the following required warning statement on the package label: “WARNING: This product contains nicotine. Nicotine is an addictive chemical” (21 C.F.R. § 1143.3(a)). It also is unlawful for such tobacco product manufacturer, packager, importer, distributor, or retailer of the tobacco product to advertise or cause to be advertised within the United States any tobacco product unless each advertisement bears the required warning statement (21 C.F.R.
§ 1143.3(b)). Under 21 C.F.R. § 1140.3, a “covered tobacco product” is defined as any tobacco product deemed to be subject to the FD&C Act under 21 C.F.R. § 1100.2, excluding components or parts not made or derived from tobacco. Before 21 C.F.R. § 1100.2 became effective, only cigarettes, smokeless tobacco, roll-your-own tobacco, and cigarette tobacco were subject to chapter IX of the FD&C Act. 21 C.F.R. § 1100.2 deems all other tobacco products, except accessories of such tobacco products, subject to chapter IX and its implementing regulations. The products cited in this violation are “covered tobacco products.” Under section 903(a)(7)(B) of the FD&C Act (21 U.S.C. § 387c(a)(7)(B)), tobacco products are misbranded if sold or distributed in violation of regulations prescribed under section 906(d) of the FD&C Act, including those within 21 C.F.R. Part 1143. Because labeling and/or advertising in the social media posts on behalf of, and by, Humble Juice Co. LLC regarding these e-liquid products does not include the required nicotine warning statement for these products, in violation of 21 C.F.R. § 1143.3(a) and/or 21 C.F.R. § 1143.3(b), the e-liquid products are misbranded under section 903(a)(7)(B) of the FD&C Act (21 U.S.C. § 387c(a)(7)(B)).
In addition, a tobacco product is misbranded under section 903(a)(1) of the FD&C Act (21 U.S.C. § 387c(a)(1)) if its labeling is false or misleading in any particular. A tobacco product is misbranded under section 903(a)(7)(A) of the FD&C Act (21 U.S.C. § 387c(a)(7)(A)) if, in the case of any tobacco product distributed or offered for sale in any State, its advertising is false or misleading in any particular. Under section 201(n) of the FD&C Act (21 U.S.C. § 321(n)), in determining whether labeling and/or advertising is misleading, the agency considers, among other things, the failure to reveal material facts concerning the consequences that may result from the customary or usual use of the product. Because labeling and/or advertising in the social media posts on behalf of, and by, Humble Juice Co. LLC regarding these e-liquid products does not include the required nicotine warning statement for these products, the e-liquid products are misbranded under section 903(a)(1) of the FD&C Act (21 U.S.C. § 387c(a)(1)) and/or section 903(a)(7)(A) of the FD&C Act (21 U.S.C. § 387c(a)(7)(A)).
The violations discussed in this letter do not necessarily constitute an exhaustive list. You should immediately correct the violations referenced above, as well as violations that are the same as or similar to those stated above, and take any necessary actions to bring your tobacco products into compliance with the FD&C Act.
It is your responsibility to ensure that your tobacco products and all related labeling and/or advertising on your website, on any other websites (including e-commerce, social networking, or search engine websites), and in any other media in which you advertise comply with each applicable provision of the FD&C Act and FDA’s implementing regulations. Failure to ensure full compliance with the FD&C Act may result in FDA initiating further action without notice, including, but not limited to, civil money penalties, no-tobacco-sale orders, criminal prosecution, seizure, and/or injunction. Please note that any adulterated or misbranded tobacco products offered for import into the United States are subject to detention and refusal of admission.
Unfair or Deceptive Marketing
The FTC has reviewed the social media postings concerning these Humble Juice e-liquid products. Section 5 of the FTC Act, 15 U.S.C. § 45, prohibits unfair or deceptive acts or practices in or affecting commerce. This prohibition includes the failure to disclose material health or safety risks. See Swisher Int’l, Inc., 2000 WL 1207447 (MSNET Aug. 25, 2000) (consent order requiring health warnings for cigar packages and advertisements); Lorillard, 80 F.T.C. 455 (1972) (consent orders requiring health warnings in cigarette advertisements). The e-liquid products cited in the above-referenced social media postings contain nicotine. The U.S. Surgeon General has long recognized the addictive nature of tobacco products due to the presence of nicotine. See U.S. Dept. of Health and Human Services, “The Health Consequences of Smoking: Nicotine Addiction,” A Report of the Surgeon General, 1988. Given the significant risk of addiction, the failure to disclose the presence of and risks associated with nicotine raises concerns that the social media postings could be unfair or likely to mislead consumers. The FTC urges you to review your marketing, including endorsements by your social media influencers, and ensure that necessary and appropriate disclosures are made about the health risks of nicotine.
In addition, the FTC’s Guides Concerning Use of Endorsements and Testimonials in Advertising, 16 C.F.R. § 255.5 (2018) (Endorsement Guides), state that if there is a “material connection” between an endorser and the marketer of a product – in other words, a connection that might affect the weight or credibility that consumers give the endorsement – that connection should be clearly and conspicuously disclosed, unless the connection is already clear from the context of the communication containing the endorsement. Material connections could consist of a business, family, or personal relationship; monetary payment; or the provision of free products to the endorser. The Endorsement Guides apply to marketers and endorsers. FTC staff guidance makes clear that marketers should advise endorsers of their disclosure responsibilities and should monitor their endorsements to ensure that appropriate disclosures are made.
If your company has a material connection to someone endorsing your products, that relationship should be clearly and conspicuously disclosed in the endorsements, unless the relationship is otherwise apparent. To be both “clear” and “conspicuous,” the disclosure should use unambiguous language and stand out. Consumers should be able to notice the disclosure easily, and not have to look for it. For example, consumers viewing posts in their Instagram streams on mobile devices typically see only the first two lines of a longer post unless they click “more,” and many consumers may not click “more.” Therefore, an endorser should disclose any material connection above the “more” button. In addition, where there are multiple tags, hashtags, or links, readers may just skip over them, especially where they appear at the end of a long post.
If your company has a written social media policy that addresses the disclosure of material connections by endorsers, you may want to evaluate how it applies to the posts identified in this letter and to posts by other endorsers of your products. If your company does not have such a policy, you may want to consider implementing one that provides appropriate guidance to your endorsers. You may also want to review your company’s social media marketing to ensure that posts contain necessary disclosures and they are clear and conspicuous.
Conclusion and Requested Actions
With regard to the FDA-related violations described in this letter, please submit a written response to this letter within 15 working days from the date of receipt describing your corrective actions, including the dates on which you discontinued the violative labeling, advertising, sale, and/or distribution of these tobacco products and your plan for maintaining compliance with the FD&C Act. If you do not believe that your products are in violation of the FD&C Act, include your reasoning and any supporting information for our consideration. You can find the FD&C Act through links on FDA’s homepage at http://www.fda.gov.
Please note your reference number, RW1901097, in your response and direct your response to the following address:
DPAL-WL Response, Office of Compliance and Enforcement
FDA Center for Tobacco Products
c/o Document Control Center
Building 71, Room G335
10903 New Hampshire Avenue
Silver Spring, MD 20993-0002
If you have any questions about the content of this letter, please contact Ele Ibarra-Pratt at (301) 796-9235 or via email at CTPCompliance@fda.hhs.gov.
With regard to the FTC-related issues described in this letter, please notify Rosemary Rosso of the FTC via electronic mail at rrosso@ftc.gov within 15 days of receipt of this letter of the specific actions you have taken to address the FTC’s concerns.
Sincerely,
/S/
Ann Simoneau, J.D.
Director
Office of Compliance and Enforcement
Center for Tobacco Products
/S/
Mary K. Engle, J.D.
Associate Director
Division of Advertising Practices
Federal Trade Commission
VIA UPS and Electronic Mail
cc:
Humble Juice Co LLC
Attn: Daniel Clark
2535 Conejo Spectrum St, Building 4C
Thousand Oaks, CA 91320
Domains By Proxy, LLC
humblejuiceco.com@domainsbyproxy.com
GoDaddy.com, LLC
Shopify, Inc.