FDA Spearheads Global Efforts on Informal Markets and Substandard/Falsified Medical Products
FROM A GLOBAL PERSPECTIVE
By Harinder Chahal and Steph Tan, FDA Office of Economics and Analysis
January 13, 2025
Harinder Chahal, PharmD, MSc, is Director of the Public Health Strategy and Analysis Staff in the FDA’s Office of Economics and Analysis (OEA). He is the U.S. Chair for the Informal Markets Workgroup for the World Health Organization’s Member State Mechanism on Substandard and Falsified Medical Products. Steph Tan, also with OEA, is a Public Health and Regulatory Research Fellow. Both Dr. Chahal and Ms. Tan recently participated in the November meeting of the Member State Mechanism in Geneva, Switzerland.
Much of the world experiences threats posed by the distribution of substandard and falsified (i.e., poor-quality and fake) medical products through informal (unregulated) markets. The public health impacts of these substandard and falsified medical products (SF or SFMPs) range from insufficient effectiveness to adverse events, including death. Informal markets exist everywhere — in the United States and abroad — in both physical (e.g., street vendors) and virtual (i.e., internet, social media, etc.) forms. Medications and other health products distributed in these informal settings are of unknown quality and could be substandard or falsified.
Regulators and other officials need the ability to effectively respond to SFMPs, especially in a global society that is increasingly reliant on the internet for buying medical products. Responses to this crisis will vary globally, as the characteristics of informal markets and the impact of SF medical products differ significantly across regions and jurisdictions. For example, nations vary in their internet use for virtual informal markets and magnitude of physical informal markets, as well as access to health care and pharmacies (particularly in rural areas), health system structures, and capacity to regulate medical products — all factors that can influence the availability and use of medical products from informal markets.
However, the scarcity of evidence on the scope and prevalence of informal markets, including distribution of SFMPs in these unregulated sectors, poses a major challenge for global health policymakers and national regulatory agencies. In fact, these knowledge gaps severely limit public health authorities from intervening to minimize the risks of SFMPs from informal markets.
Despite this daunting reality, there is cause for optimism. The United States — a founding member of the World Health Organization’s Member State Mechanism on Substandard and Falsified Medical Products (WHO Mech) — alongside the 194 countries that are part of this forum, plays a substantial role in advancing intergovernmental efforts to mitigate the global proliferation of SF medical products, with a keen focus on informal markets for medical products.
In October 2021, the United States put forth a proposal to the members of the WHO Mech to create a technical workgroup that would focus on the distribution of SF medical products through informal markets. Recognizing the importance of the issue that permeates all countries, the WHO Mech, by unanimous consent, established the Informal Markets Working Group (IMWG) with the mandate to “develop strategies for national regulatory authorities to mitigate the public health risks posed by the distribution of substandard and falsified medical products through informal markets.” The IMWG, chaired by the United States, now consists of 20 countries from five WHO regions.[1]
Identifying the Threat
Following its launch in 2021, the IMWG developed four high-level thematic areas under which it now conducts specific technical activities. These areas include: 1) defining “informal markets” as they relate to medical products; 2) understanding the existing knowledge base and knowledge gaps on informal markets for medical products; 3) gathering evidence to address knowledge gaps in informal markets; and 4) developing strategies and policy recommendations to help countries combat the distribution of SF medical products via informal markets.
The strides made by the IMWG within these thematic areas have been significant. As its initial act, the IMWG developed the first definition of informal markets for medical products. The definition was adopted by the WHO Mech in 2022 and now serves as a global functional definition. According to this definition, an informal market is a sector of national or local economy where:
- The manufacture, import or export, distribution, sale, supply, or purchase of medical products takes place outside of the legal, regulatory, or administrative oversight of relevant public health or regulatory authorities.
- The medical products have or have not been assessed for safety, efficacy or quality by public health and regulatory authorities (authorized products found on the informal market are not considered substandard and falsified products per se).
- The activities mentioned above may be conducted by persons or entities with or without appropriate qualifications and may take place in a physical, virtual, or hybrid environment.
Additionally, the IMWG completed two technical activities in 2023 under theme No. 2 (understanding the current knowledge base and knowledge gaps on informal markets for medical products). The first was a comprehensive literature review of scientific publications over the last 20 years on informal markets for medical products with a focus on SF medical products. The review (a summary is available on the WHO website), which included causes for the distribution of medical products via informal markets, also incorporated research on known public health and economic impacts, alongside existing mitigation efforts. The fundamental finding of this review was that to address informal markets for medical products, a standardized methodology, “that is adaptable for country-specific contexts, in the form of a toolkit to provide Member States with the necessary tools to systematically gather country-level evidence on the various aspects of physical and online informal markets” is needed.
The second technical activity, a pilot survey of 20 Member States, was conducted to further the understanding of existing knowledge — and related intelligence gaps — of informal markets for medical products from the viewpoint of national regulatory authorities. Though completed by only 11 countries (55% response rate), the survey reinforced that most countries have limited knowledge of the informal markets within their jurisdictions and are requesting tools and technical guidance to better understand both physical and virtual informal markets. As an immediate next step, the IMWG plans to build on this effort by conducting a broader, comprehensive survey of all 194 countries in the WHO Mech. The aim is to gain deeper insights into what countries currently understand about their informal markets and identify areas where they seek further knowledge.
The Need for a Research Methodology Toolkit
Most recently, in November 2024, following extensive consultation from global experts, the IMWG made inroads under the third theme – gather evidence to address knowledge gaps in informal markets. The IMWG received the WHO Mech consensus on a framework for developing a first-of-its-kind research methodology toolkit that can be tailored to better understand informal markets and SFMPs to fill knowledge gaps in each Member State.
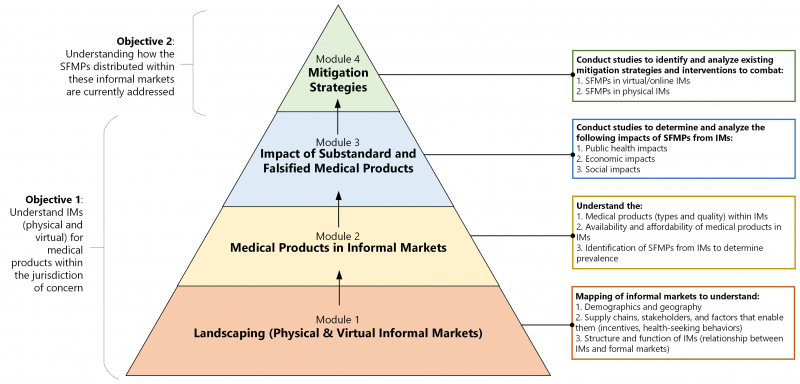
The foundation of the toolkit will be four technical modules designed to allow regulatory authorities to understand the scope and impact of informal markets in their jurisdiction and how SF medical products are currently being addressed.
The toolkit framework (see below) begins with a landscape analysis to gain baseline knowledge — this will involve the investigation of the structure and function of informal markets (what these markets look like and how they operate), the demographics and geography of these markets (who the buyers and sellers are, and where they are located both physically and virtually), and the supply chains, stakeholders, and their driving factors (what incentivizes informal market actors).
Once these informal markets are well understood, the medical products distributed within these markets can be studied, including the types and prevalence of SFMPs, followed by an assessment of the public health, economic and social impacts of the SF products. Lastly, a jurisdiction’s existing interventions and mitigation strategies for SF medical products from informal markets will be analyzed to determine their relative effectiveness and any lessons learned that could be helpful to other jurisdictions.
Due to these new developments, the years ahead will be an exciting time for the IMWG. The two immediate next steps important for the success of the toolkit are: first, establish a committee of global experts to develop the technical modules, and second, secure funding to facilitate the development, piloting, and finalization of the toolkit. Once implemented, the country-led evidence generated can be used to meaningfully address the distribution of SFMPs through informal markets by communicating research findings to the public, regulators, and policymakers across the globe. This much-needed boost in knowledge will help both the WHO and Member States to reduce the threat of high-risk, SF medical products.
[1]WHO African Region: Botswana, Ghana, Guinea, Liberia, Niger, Nigeria, South Africa; WHO Americas Region: Brazil, El Salvador, Nicaragua, Panama, United States of America; WHO Eastern Mediterranean Region: Iraq; WHO South-East Asia Region: India, Indonesia, Nepal; WHO Western Pacific Region: Cambodia, China, Malaysia, Republic of Korea