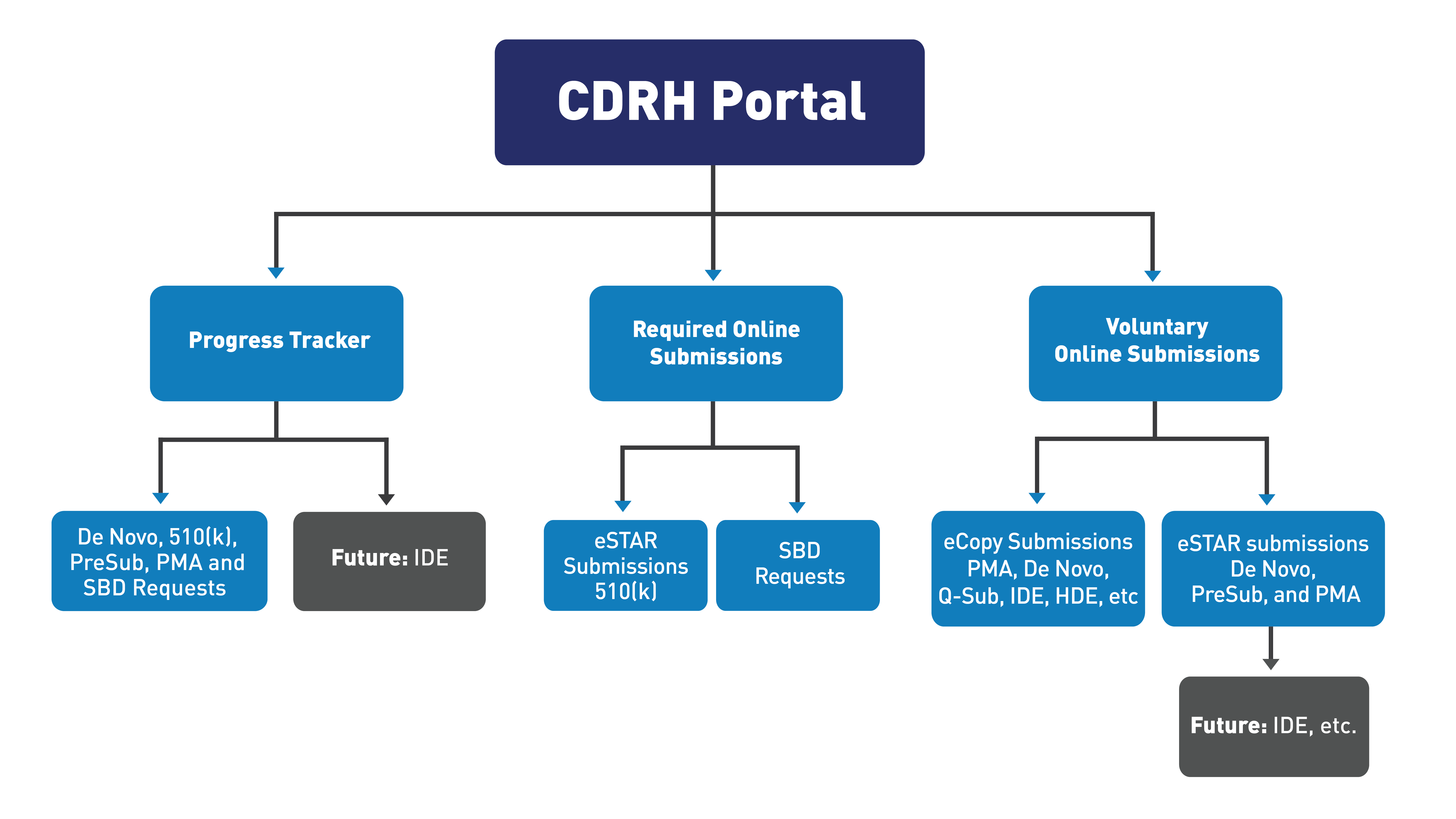
Send and Track Medical Device Premarket Submissions Online: CDRH Portal
Update: October 3, 2024
The U.S. Food and Drug Administration (FDA) updated CDRH’s Customer Collaboration Portal (CDRH Portal) to:
- Accept Small Business Determination (SBD) requests. Details about this process are included below on this page. Additional information about the small business eligibility and submission requirements is available on the Small Business Determination (SBD) Program webpage.
- Track the progress of Premarket Approval (PMA) applications and PMA Panel-Track supplements in the CDRH Portal. Users can view their application status in near real-time and review milestones.
The CDRH Portal updates for PMAs are a step forward in meeting the Medical Device User Fee Amendments 2022 (MDUFA V) commitments of using technology to enhance efficiency and transparency in reviewing industry submissions.
All CDRH-led premarket submission types may be uploaded to the CDRH Portal at any stage of the review process. Official correspondents do not need to send a physical cover letter to the FDA after uploading an electronic submission to the CDRH Portal. Please note that the CDRH Portal cannot receive submission files larger than 4GB or PDF files with attachments larger than 1GB.
On this page:
- CDRH Portal: Send Medical Device Premarket Submissions and Small Business Determination Requests Online
- Progress Tracker for Electronic Submissions
- Technical Limitations
- Next Steps
- For More Information
CDRH Portal: Send Medical Device Premarket Submissions and Small Business Determination Requests Online
Anyone can register for an account in the CDRH Portal and send premarket submissions and Small Business Determination requests online.
Important: CDRH uses Okta for identity verification and single sign-on purposes when users register for a CDRH Portal account. Account registration and password reset requests will come from an email ending in @okta.com.
Beginning November 1, 2024, all Small Business Determination requests must be submitted as electronic submissions using the CDRH portal.
As of October 1, 2023, all 510(k) submissions, unless exempted*, must be submitted as electronic submissions using eSTAR.
*As noted in the final guidance, Electronic Submission Template for Medical Device 510(k) Submissions: Guidance for Industry and FDA Staff, all 510(k)s, including Traditional, Special, Abbreviated, and any supplements and amendments are required to be submitted as electronic submissions, unless exempted in Section VI.A Waivers and Exemptions from Electronic Submission Requirements of the guidance. The electronic submission template, eSTAR, is the only currently available electronic submission template to facilitate the preparation of 510(k) electronic submissions.
Submissions and requests received before 4 PM ET on a business day (Monday through Friday, excluding U.S. Federal holidays) will be processed the same day. If received after 4PM ET, the submission will be processed the next business day.
NOTE: Only CDRH submissions may be sent through the CDRH Portal. For biologic products or devices used in blood establishments, please submit to the Center for Biologics, Evaluation, and Research (CBER).
Progress Tracker for Electronic Submissions
The online progress tracker has a dashboard that displays near real-time submission status. The FDA secures the information about each submission's progress to ensure only the official correspondent or designated delegates* for that submission can view the status.
When you send a CDRH Pre-Submission, 510(k) submission (traditional, special, and abbreviated 510(k)s), De Novo classification request, Premarket Approval (PMA) application, PMA Panel-Track supplement or Small Business Determination request for review, your official correspondent or designated delegates can monitor the FDA’s progress online in a simple, concise format.
The official correspondent for an eSTAR submission is the person identified in the:
- “Contact” section, or as the
- “Primary Correspondent/ Consultant,” if applicable.
The official correspondent for an eCopy submission is the person identified in:
- Section C of CDRH Premarket Review Submission Cover Sheet (Form FDA 3514) or in
- Section B (if Section C is blank on the Cover Sheet form), or
- in the Cover letter, if the eCopy submission does not include a Cover Sheet form.
The official correspondent for a Small Business Determination Request is the person identified in:
- Section I, Boxes 4 through 7 of Form FDA 3602 and 3602A
* Note: At this time, only the official correspondent can view the status of their SBD Request. Assignment to a designated delegate will be available in a future update.
What to expect:
- If this is your official correspondent’s first time tracking a submission online, the FDA automatically emails a link to create a login password soon after the FDA starts its review.
- While the CDRH Portal features online progress tracking for Pre-Submissions, 510(k) submissions, De Novo classification requests, PMA applications, PMA Panel-Track supplements and Small Business Determination requests, the FDA also formally notifies you of your submission's status by emailing your official correspondent with official actions and requests.
Technical Limitations
The CDRH Portal cannot receive individual files that are:
- larger than 4GB, or
- PDFs with an attachment larger than 1GB.
To submit electronic files that exceed the technical limitations, you can mail the electronic version of your submission to the CDRH Document Control Center (DCC) at this address:
U.S. Food and Drug Administration
Center for Devices and Radiological Health
Document Control Center (DCC) – WO66-G609
10903 New Hampshire Avenue
Silver Spring, MD 20993-0002
For more information on mailing submissions, please read eCopy Medical Device Submissions.
Next Steps
The FDA will maintain and improve on the Customer Collaboration Portal ("CDRH Portal") as described in the Draft: Medical Device User Fee Amendments (MDUFA) Performance Goals and Procedures, Fiscal Years 2023 through 2027.
For more information:
- eCopy Program for Medical Device Submissions
- Electronic Submissions
- eSTAR Program
- How to Study and Market Your Device
- Premarket Approval
- Small Business Determination (SBD) Program
* When final, this guidance will represent FDA’s policy on this topic.
Questions?
If you have questions about:
- CDRH Portal, check the Portal Help articles inside the CDRH Portal, or email ccp@fda.hhs.gov
- eCopy, email CDRH-eCopyinfo@fda.hhs.gov
- eSTAR, email eSubPilot@fda.hhs.gov
- Device regulation in general, email DICE@fda.hhs.gov
- Specific premarket submissions, email OPEQSubmissionSupport@fda.hhs.gov
- General Small Business Determination Program information, email DICE@fda.hhs.gov