Breathing Circuit Kit Recall: Sentec/Percussionaire Removes VDR4 Phasitron Breathing Circuits due to Venturi Component Malfunctions that May Reduce Pressure and Volume Flow
This recall involves removing certain devices from where they are used or sold. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use it.
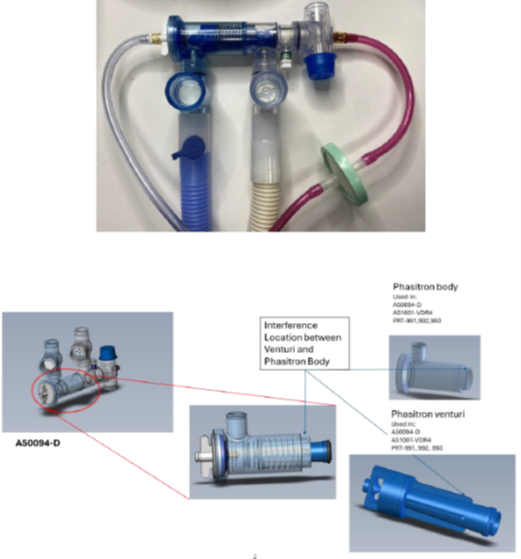
Affected Product
- Product Names/Models and Part Numbers:
- Phasitron Kit, VDR, Single Patient A50094-D, A50094-D-5PK
- Unique Device Identifier (UDI): 00849436000259
- Control Unit Tester A51001-VDR4
- VDR W/SWIVEL T SINGLE PATIENT PHASITRON PRT-991
- VDR4 HUMIDIFICATION ADAPTER KIT PRT-992
- VDR4 Humidification Kit with Cross Tee PRT-993
- Phasitron Kit, VDR, Single Patient A50094-D, A50094-D-5PK
- Lot/Serial Numbers: See full list of affected devices below.
What to Do
- Closely monitor all patients who are ventilated with Phasitron breathing circuits that may be affected by this issue.
- Make sure the venturi component of the Phasitron breathing circuit in use is moving by looking for movement and listening for noise.
- Do not use any Phasitron breathing circuit that is malfunctioning or does not pass the enhanced pre-check instructions outlined below.
On December 23, 2024, Sentec/Percussionaire sent all affected customers an Urgent Medical Device Notification recommending the following actions:
- Make sure all patients are closely monitored using pulse oximetry and/or carbon dioxide monitoring to identify any changes in patient condition caused by impaired Phasitron function.
- Look and listen for venturi movement. The venturi component produces a loud noise and can be seen opening and closing through the translucent Phasitron body when functioning properly. A lack of visible or audible motion indicates device malfunction.
- Immediately stop use if any product malfunction is identified during use.
- Dispose according to institutional protocol.
- Contact FSCA@sentec.com for product exchange.
- Review all inventory for impacted lots.
- Dispose of product from affected lots once replacement products are received.
- Evaluate the circuits of Phasitron breathing circuit kits in identified lots using the enhanced pre-use check outline below, also included in Appendix 2 of the letter.
- Post pre-check instructions in all areas of the facility.
- Do not use any product that fails the pre-use check outlined below.
- Dispose of any failing product using institutional protocol.
- Contact FSCA@sentec.com for product exchange.
- Keep an alternative device nearby in case of a Phasitron breathing circuit failure.
- Complete and Return Acknowledgement form included with the letter by January 31, 2024, and after reviewing and implementing the requested actions.
- Report adverse events to regulatory.percussionaire@sentec.com and/or to the FDA’s MedWatch Adverse Event Reporting program.
- Report any quality problems experienced with the use of this product to Percussionaire/Sentec Customer Service department via email to FSCA@sentec.com.
Sentec/Percussionaire Pre-Use Check Instructions
Pre-use check must be completed before any ventilation is started on a new patient, when a new circuit is used, and after each circuit cleaning. If any abnormal function is noted, do not start ventilation.
- Connect hospital air supply hose to VDR-4. Listen for blender alarm, then disconnect air hose.
- Connect hospital oxygen supply hose to VDR-4 and disconnect air hose. Listen for blender alarm.
- Connect hospital air supply hose to VDR-4.
- Turn Monitron II “ON.”
- Connect Phasitron patient port to a test lung (such as Vadi 210 or equivalent).
- Perform all tests using standard heater/humidification setup, set up according to hospital protocol.
- Turn operating pressure knob until it reaches a static 42 psig.
- Turn VDR-4 “ON.”
- Turn off OSCILLATORY PEEP/CPAP, DEMAND CPAP, and CONVECTIVE PRES. RISE (full clockwise).
- Set the PULSATILE FLOWRATE control to AIP of 30 cmH₂O as read on DM or manometer.
- Set pulse frequency to 500.
- Set PULSE i/e RATIO with arrow at the 12:00 position (straight up) for a 1:1 i/e ratio.
- Set inspiratory time and expiratory time to:
- 2.0 seconds to get a convective rate of ~15 (adult/large peds)
- 1.5 seconds to get a convective rate of ~20 (small peds)
- 1.0 second to get a convective rate of ~30 (neonatal)
- Set oscillatory CPAP/PEEP to AEP of 5 cmH₂O as read on Digital Multimeter (DM) or manometer.
- Check Monitron II for appropriate rise and fall on waveform.
- Verify pulse frequency will go greater than 700 and less than 200.
- Return pulse frequency to 500.
- Manually compress test lung and hold as tightly as possible.
- Verify pulsatile flowrate will achieve AIP of 50 cmH₂O.
- Verify oscillatory CPAP will reach a minimum AEP of 18 cmH₂O. (Release hold on test lung).
- Return pulsatile flowrate to an AIP of 30 cmH₂O and OSCILLATORY CPAP control to an AEP of 5 cmH₂O.
- Verify a gradient of 8-10 cmH₂O when convective pressure rise is applied.
NOTE: Convective pressure rise will begin after approximately 0.7 seconds have passed from the start of the inspiration cycle. - Increase convective pressure rise until failsafe alarm sounds. Observe that ventilation continues at lower settings. Turn convective pressure rise off and then press red button to reset.
- Remove the test lung. Cap both Phasitron ports.
- Set the PULSATILE FLOWRATE knob fully to the right to the “Off” position.
- Set the OSCILLATORY CPAP/PEEP knob fully to the left to the “On” position.
- Turn DEMAND CPAP/PEEP knob to the 12:00 position (arrow up) and allow at least 15 seconds for the Digital Multimeter (DM) to switch to Active mode.
- Reduce the DEMAND CPAP/PEEP to achieve a Mean Airway Pressure (MAP) of 3-4 cmH2O according to the DM. Observe that the DM remains on and in Active mode.
- While observing the Venturi in the Phasitron, slowly turn the PULSATILE FLOWRATE knob very slightly to the left to achieve a MAP of 4-5 cmH2O. The Pulse Frequency should be displayed on the DM.
- Ensure that the Pulse Frequency Rate is 500-600 Cycles Per Minute (CPM). Adjust if needed using the PULSE FREQUENCY knob only.
- Observe the Venturi for 5 seconds or more. Look for oscillations of the Venturi. If the Venturi is not moving or moving intermittently and erratically, discontinue use of the circuit and replace it with another circuit.
- Lower high-amplitude pressure alarm below set pulsatile flowrate to trigger the high-pressure alarm. Press reset on Monitron to clear audible alarm.
- Trigger low-amplitude pressure alarm by disconnecting test cap. Press reset on Monitron II to clear audible alarm.
- Turn off VDR-4 and Monitron II. Silence alarm on Monitron II by pressing any button.
- Switch nebulizer on and listen for gas flow, then switch off.
Reason for Recall
Sentec/Percussionaire is recalling Phasitron breathing circuit kits after receiving a customer complaint that the venturi component of the kit stopping moving (oscillating) during use. The issue caused a patient’s oxygen levels to fall (desaturate). The risk of failure is especially high for pediatric patients due to the low pressures that are associated with this component failure.
The use of affected product may cause serious adverse health consequences, including acute respiratory failure, hypoxia (lack of oxygen), hypercapnia (excess carbon dioxide in the blood), potential brain damage, heart complications, increased risk of pneumonia, and death.
There have been no reported injuries. There have been no reports of death.
Device Use
The Phasitron breathing circuit kit is intended to be used for continuous, controlled ventilation of patients who are unable to breathe on their own. The venturi component of the Phasitron helps with the pulsing flow of air/oxygen to the patient.
Contact Information
Customers in the U.S. with questions about this recall should contact Sentec/Percussionaire customer service at FSCA@sentec.com.
Full List of Affected Devices
Part Number/ Product Name:
- A50094-D-5PK - Phasitron Kit, VDR, Single Patient, 5pk
- A51001-VDR4 - Control Unit Tester
- PRT-991 - VDR W/SWIVEL T SINGLE PATIENT PHASITRON
- PRT-992 - VDR4 HUMIDIFICATION ADAPTER KIT, CASE OF 10
- PRT-993 - VDR4 Humidification Kit with Cross Tee, Case of 10
Potential Affected Lots:
- A50094-D-5PK: WO04294, WO04424, WO04764, WO05070, WO05186, WO05460, WO05685, WO05910, WO06388, WO06576, WO06883, WO07095, WO07196, WO07317, WO07405, WO07450, WO07696
- A51001-VDR4: WO04750, WO06701
- PRT-991: WO04733
- PRT 992: WO045667
- PRT-993: WO04745, WO04832, WO04893, WO04893, WO05309, WO06523, WO07080, WO07283, WO04893
Additional FDA Resources
Unique Device Identifier (UDI)
The unique device identifier (UDI) helps identify individual medical devices sold in the United States from manufacturing through distribution to patient use. The UDI allows for more accurate reporting, reviewing, and analyzing of adverse event reports so that devices can be identified, and problems potentially corrected more quickly.
- How do I recognize a UDI on a label?
- AccessGUDID database - Identify Your Medical Device
- Benefits of a UDI System
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program.