Early Alert: Aspiration System Issue from Calyxo
This communication is part of the Communications Pilot to Enhance the Medical Device Recall Program. The FDA has become aware of a potentially high-risk device issue. The FDA will keep the public informed and update this web page as significant new information becomes available.
Affected Product
The FDA is aware that Calyxo has issued a letter to affected customers indicating CVAC Aspiration Systems have updated instructions for use:
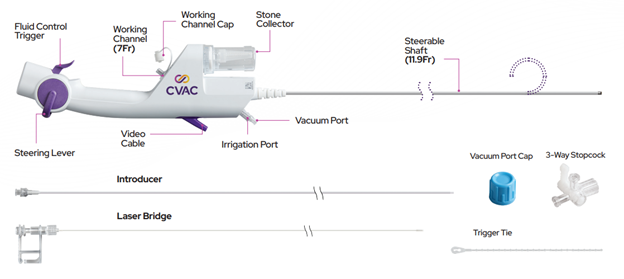
- CVAC Aspiration System
- Calyxo REF Number CVC127020-1 and User Manual L00018 Rev C
- UDI-DI: 00860005357710
What to Do
- On February 19, 2025, Calyxo sent all affected health care providers an Urgent Device Field Correction notice recommending the following actions:
- Notify and provide all urologists using the CVAC Aspiration System in your institution that additional instructions are available and are required.
- Do not continue to provide fluid inflow in the presence of unresolved slow or absent fluid outflow. Doing so can create an intrarenal pressure imbalance, which may result in serious injury or death.
- If a patient has cloudy, opaque (turbid), or suspected thick (high-viscosity) fluid observed in the kidney’s collecting system, stop irrigation immediately using the three-way stopcock.
- If visibility within the collecting system is completely obscured by opaque fluid, do not use the CVAC Aspiration System in the procedure.
- Diagnostic or therapeutic ureteroscopy is contraindicated in patients with untreated urinary tract infection. Patients with coagulation disorders, severe cardiopulmonary insufficiency, or uncontrolled diabetes should be managed appropriately.
- If drainage through the access sheath is desired, use a 13/15 Fr or larger Ureteral Access Sheath. The CVAC Aspiration System is compatible with 12/14 Fr Ureteral Access Sheaths and will provide limited outflow, which may over-pressure the kidney.
- Confirm your equipment is set up properly for use with the CVAC Aspiration System to monitor fluid outflow when low outflow is suspected. If there is a suspected slowing or lack of fluid outflow from the CVAC Aspiration System, stop irrigation inflow using the three-way stopcock.
- If visibility is sufficient to proceed, follow the evacuation procedure to clear the fluid in the kidney.
- Notify and provide all urologists using the CVAC Aspiration System in your institution that additional instructions are available and are required.
- Check this web page for updates. The FDA is currently reviewing information about this potentially high-risk device issue and will keep the public informed as significant new information becomes available.
Reason for Early Alert
Calyxo has identified a new risk of injury during use of the CVAC Aspiration System when patients have thick (high viscosity) fluid in the kidney at the start of the procedure, which can cause reduced fluid outflow that can lead to excessive pressure in the kidney. If the increased pressure in the kidney is not addressed, serious injury or death may occur.
Calyxo has reported 1 death associated with this issue.
Device Use
The CVAC System (CVAC Aspiration System and CVAC Image Processor) is used to establish a channel during endoscopic urological procedures for the treatment and removal of urinary stones.
Contact Information
Customers in the U.S. with adverse reactions, quality problems, or questions about this recall should contact Calyxo at qualitycontrol@calyxoinc.com or 833-214-3354.
Unique Device Identifier (UDI)
The unique device identifier (UDI) helps identify individual medical devices sold in the United States from distribution to use. The UDI allows for more accurate reporting, reviewing, and analyzing of adverse event reports so that devices can be identified more quickly, and as a result, problems potentially resolved more quickly.
- How do I recognize a UDI on a label?
- AccessGUDID database - Identify Your Medical Device
- Benefits of a UDI System
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program.