Endoscopic Vessel Harvesting (EVH) System Correction: Getinge and Maquet Cardiovascular Update Use Instructions for VasoView HemoPro 2 (VH-4000 and VH-4001) EVH Systems due to Risk for Bent or Detached Heater Wires and Silicone Peeling or Detaching During Use
The devices described in this recall are included in the related Letter to Health Care Providers. See the Letter to Health Care Providers for the most current information on these devices.
This recall involves updating instructions for using devices, and does not involve removing them from where they are used or sold. The FDA has identified this as a Class I recall, the most serious type of recall. This device may cause serious injury or death if you continue to use it without following the recall instructions.
Affected Product
- Product Names:
- VasoView HemoPro 2 Endoscopic Vessel Harvesting System, VH-4000
- Vasoview Hemopro 2 (w/Vasoshield) Endoscopic Vessel Harvesting System, VH-4001
- Model Unique Device Identifier (UDI)/Model:
- 00607567700406/VH-4000
- 00607567700451/VH-4001
- Lot/Serial Numbers: All unexpired lots.
What to Do
- Review Instructions for Use before using Vasoview HemoPro 2 EVH devices.
- Read FDA’s Letter to Health Care Providers related to these devices.
In December 2024, Getinge sent all affected customers an Urgent Medical Device Correction letter recommending the following actions:
- Review the safety information provided in the letter.
- Pay special attention to the following warnings from the Instructions for Use (IFU) to minimize over-delivery of energy.
- When inserting or retracting the Harvesting Tool through the Harvesting Cannula, close the Jaws and ensure the Jaw tips are oriented upwards (concave side up) to prevent damage to the Jaws.
- Application of energy without tissue between the Jaws of the Harvesting Tool should be kept to minimum in order to maximize Harvesting Tool performance.
- When separation of the branch tissue is noticed, open the Jaws and stop application of energy by pushing the Activation Toggle into the forward most position and retract the Harvesting Tool slightly.
- NOTE: Once material has been transected, stop application of energy.
- Consider the following actions to mitigate risks:
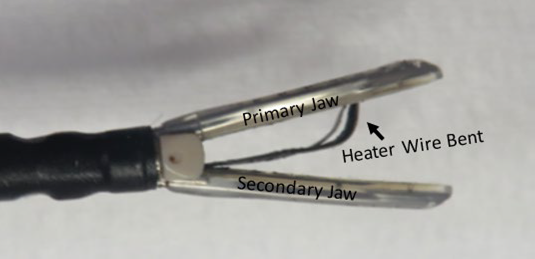
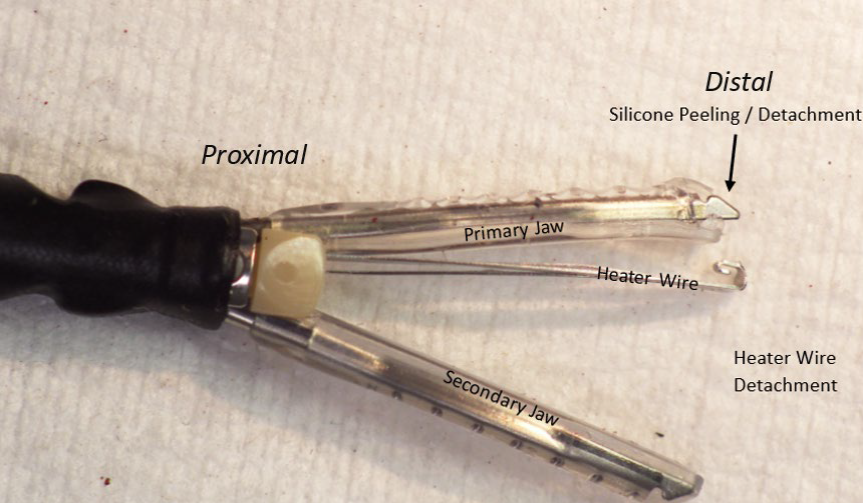
- Inspect the device prior to use for any signs of damage including silicone peeling away from the jaws.
- Check the outer surface of the device for rough surfaces, sharp edges, or unusual protrusions that may be a hazard.
- Monitor the device during use for silicone peeling away from the jaws.
- Inspect the device after use for missing or damaged parts.
- Stop using the device if, at any time of use, there are missing or damaged parts or peeling of silicone.
- Locate and remove any fragmented components from the patient.
- Monitor patients for complications if you suspect fragment(s) of the device may have been retained. Future complications could include delayed onset of pain, infection, and/or localized allergic/adverse reaction.
- Complete and sign the Medical Device Removal Response Form attached to the letter.
- Return the completed form to Maquet Cardiovascular/Getinge by email:
Hemopro-peelinq-detachedsilicone2024.act@getinge.com or fax: 1-866-594-8101. - Forward this information to all current and potential device users within your hospital/facility.
- Distributors should forward to any customers who may have received this product.
Reason for Recall
Getinge and its subsidiary Maquet Cardiovascular are recalling VasoView HemoPro 2 (VH-4000 and VH-4001) Endoscopic Vessel Harvesting Systems due to the possibility of two failure modes experienced during use: a bent or detached heater wire and silicone peeling or detaching from the jaws of the harvesting tool.
The use of affected product may cause serious adverse health consequences, including bleeding, burns, injury, or blockage (embolism or occlusion) of blood vessels, infection, and death.
There have been seven reported serious injuries, four for the heater wire and three for silicone peeling or detaching. There have been no reports of death.
Device Use
VasoView HemoPro 2 Endoscopic Vessel Harvesting (EVH) Systems are indicated for use in minimally invasive surgery, allowing access for vessel harvesting. These systems are used for patients undergoing endoscopic surgery to create a new path for blood flow in the arteries (arterial bypass).
Contact Information
Customers in the U.S. with questions about this recall should contact their Maquet Cardiovascular/Getinge representative or call Maquet Cardiovascular/Getinge Customer Support at 1-888-880-2874.
Additional FDA Resources
- FDA Communication [12/9/2024]: Safety and Availability Concerns with VasoView HemoPro Endoscopic Vessel Harvesting Systems - Letter to Health Care Providers
- FDA’s Enforcement Report
- Medical Device Recall Database
Additional Company Resources
Company provided information on a recall is posted here by the FDA as a public service.
Unique Device Identifier (UDI)
The unique device identifier (UDI) helps identify individual medical devices sold in the United States from manufacturing through distribution to patient use. The UDI allows for more accurate reporting, reviewing, and analyzing of adverse event reports so that devices can be identified, and problems potentially corrected more quickly.
- How do I recognize a UDI on a label?
- AccessGUDID database - Identify Your Medical Device
- Benefits of a UDI System
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program.