AAV5 DetectCDx – P190033
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: AAV5 DetectCDx
PMA Applicant: ARUP Laboratories
Address: 500 Chipeta Way, Salt Lake City, UT 84108
Approval Date: June 29, 2023
Approval Letter: Approval Order
What is it?
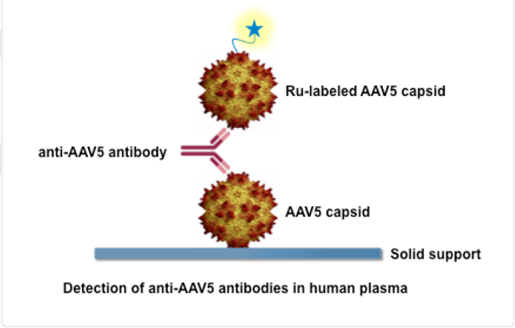
The AAV5 DetectCDx is a laboratory test that detects antibodies to the virus, adeno-associated virus serotype 5 (AAV5), a virus people are commonly exposed to in the environment. This test helps doctors identify adults with severe hemophilia A who do not have pre-existing antibodies to AAV5 and who may benefit from personalized treatment with the AAV5-based gene therapy ROCTAVIAN (valoctocogene roxaparvovec-rvox).
How does it work?
A plasma sample from a person with severe hemophilia A is sent to ARUP Laboratories for testing. The plasma sample is mixed with chemical substances, called reagents, that detect antibodies to the type of adeno-associated virus (AAV5) that is used to deliver the ROCTAVIAN gene therapy. If the test does not detect these AAV5 antibodies, the person is presumed to be AAV5 antibody negative and may benefit from treatment with ROCTAVIAN.
A medical professional reviews the results and sends a report to the doctor who ordered the laboratory test. The doctor uses this information to help create a treatment plan for a person who is already diagnosed with severe hemophilia A.
When is it used?
Doctors use the AAV5 DetectCDx to test if a patient with severe hemophilia A may be eligible for treatment with ROCTAVIAN.
What will it accomplish?
People with severe hemophilia A who are evaluated with the AAV5 DetectCDx and are anti-AAV5 antibody negative (a “Not Detected” result) may be eligible for treatment with ROCTAVIAN under the supervision of a doctor.
When should it not be used?
There are no known reasons not to use this test.