Abbott Spinal Cord Stimulation (SCS) Systems – P010032/S191
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Abbott SCS System, including Prodigy, Proclaim Plus, Proclaim XR, and Eterna Systems
PMA Applicant: Abbott Medical
Address: 6901 Preston Road, Plano, TX 75024 USA
Approval Date: May 30, 2024
Approval Letter: Approval Order Statement
What is it?
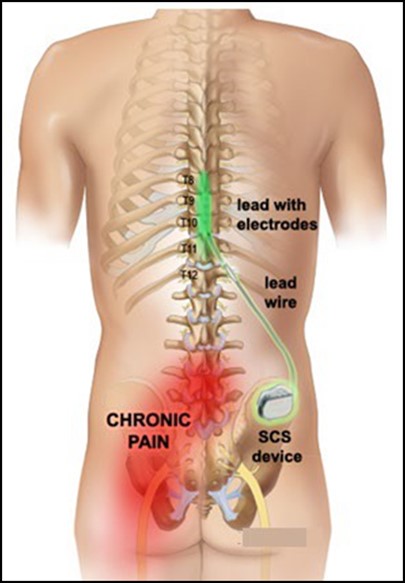
Abbott’s Spinal Cord Stimulation (SCS) System is an implantable device, called a neurostimulator, to treat long-term (chronic) pain of the torso, arms, and legs that is difficult to manage (intractable). The Abbott SCS system includes an implantable pulse generator (IPG) and lead wires, as well as the external clinician programmer and patient controller.
The Abbott SCS System can produce several types of stimulation that vary in strength, speed, and frequency. “Tonic” stimulations use a consistent stream of strong pulses, and “burst” stimulations use groups of milder and faster pulses.
This supplement specifies that both kinds of stimulation can be used to treat non-surgical back pain, but that diabetic peripheral neuropathy should only be treated with tonic stimulation.
Spinal cord stimulators deliver mild electrical stimulation to the spinal cord in order to relieve chronic, intractable pain. The signal generator device, implanted near the hip, delivers electrical pulses to the spinal cord through electrodes in the lead wires implanted near the spinal cord.
Abbott’s SCS System can aid in the management of chronic and difficult to manage pain of the torso, arms, and legs, including pain on one or both sides of the body associated with:
- Failed back surgery syndrome.
- Back pain not caused or treated with surgery and where the patient is not a candidate for surgery.
- Diabetic peripheral neuropathy (nerve damage) in the legs and feet.
The Abbott Spinal Cord Stimulation (SCS) System may help to treat chronic, difficult to manage pain of the torso, arms, and legs.
Abbott SCS system should not be used by patients who:
- Are unable to operate the system.
- Have not received effective pain relief during trial stimulation.
- Summary of Safety and Effectiveness Data (SSED)
- Labeling – Programmer User’s Guide
- Labeling Part 2 – Patient Guide
- PMA Database Entry