Edwards SAPIEN 3, SAPIEN 3 Ultra, and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System – P140031/S162
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Edwards SAPIEN 3, SAPIEN 3 Ultra, and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System
PMA Applicant: Edwards Lifesciences LLC
Address: One Edwards Way, Irvine, CA 92614
Approval Date: May 23, 2024
Approval Letter: Approval Order
What is it?
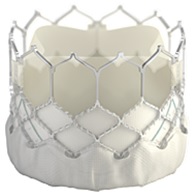
The Edwards SAPIEN 3, SAPIEN 3 Ultra, and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System (often referred to as SAPIEN 3 THV) includes a catheter-based artificial heart valve and accessories that are used to implant the valve in the heart without open-heart surgery. The valve is made of cow tissue attached to a metal (cobalt-chromium) frame for support.
This approval expands the use of this device to include using it to replace a failing, previously implanted surgical biological mitral valve (mitral valve made of cow or pig tissue), in people who are at intermediate or higher risk of complications or death from open heart surgery.
The Edwards SAPIEN 3 and SAPIEN 3 Ultra THV System was previously approved for the replacement of a failing mitral valve in patients who are too high risk for open-heart surgery when the valve has already been repaired with an artificial (prosthetic) ring or to treat severe narrowing of the aortic valve (aortic stenosis) when the valve is the patient’s original valve. It was also approved for the replacement of a failing (narrowed, leaky, or both) previously implanted surgical biological aortic or mitral valve, or transcatheter aortic valve, in patients who are too high risk for open-heart surgery.
The SAPIEN 3 THV is compressed and placed on the end of a tube-like device called a balloon catheter. The catheter is inserted through the largest vein in the leg (femoral vein) and advanced through the blood vessels to reach the failed valve. The balloon is expanded, which expands the new valve so that it attaches to the failed valve. Once in place, the new valve does a better job of opening and closing and keeps blood flowing in the right direction.
The SAPIEN 3 THV is used to replace a valve in people who already received a tissue mitral valve once before but that earlier valve is starting to narrow and/or leak, preventing blood from flowing efficiently. The heart can eventually weaken from working harder to pump blood through the valve. This can lead to life-threatening heart problems such as fainting, chest pain, heart failure, irregular heart rhythm (arrhythmia), or heart attack (cardiac arrest).
The SAPIEN 3 THV should only be used in people who have an intermediate or greater risk of serious complications or death from open-heart surgery to replace their previously implanted surgical mitral valve. A patient’s risk is determined by their medical team, including the cardiologist and surgeon.
The SAPIEN 3 THV can improve blood flow through the mitral valve in people with a failing tissue mitral valve, but who have a risk for complications or death from open heart surgery.
Clinical evidence, including a study of 499 patients who received the SAPIEN 3 THV valve, showed a reasonable assurance that the SAPIEN 3 THV is safe and effective for these patients.
Any procedure to replace the mitral valve carries the risk for serious complications. In the available clinical data of the device, the major risks observed included death, stroke, acute kidney injury, cardiac arrest, bleeding, complications with the arteries used to insert the valve, and the need for a permanent pacemaker.
For some people with other conditions or diseases, these risks may be even higher. Doctors and their patients should discuss the benefits and risks of this device together.
The SAPIEN 3 should not be used in patients who:
- cannot tolerate blood thinning medicines
- have an infection in the heart or elsewhere
- have an annuloplasty ring that is significantly detaching from the tissue (annuloplasty ring dehiscence)