Esprit BTK Everolimus Eluting Resorbable Scaffold System – P230036
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Esprit BTK Everolimus Eluting Resorbable Scaffold System
PMA Applicant: Abbott Medical
Address: 3200 Lakeside Drive, Santa Clara, CA 95054 USA
Approval Date: April 26, 2024
Approval Letter: Approval Order
What is it?
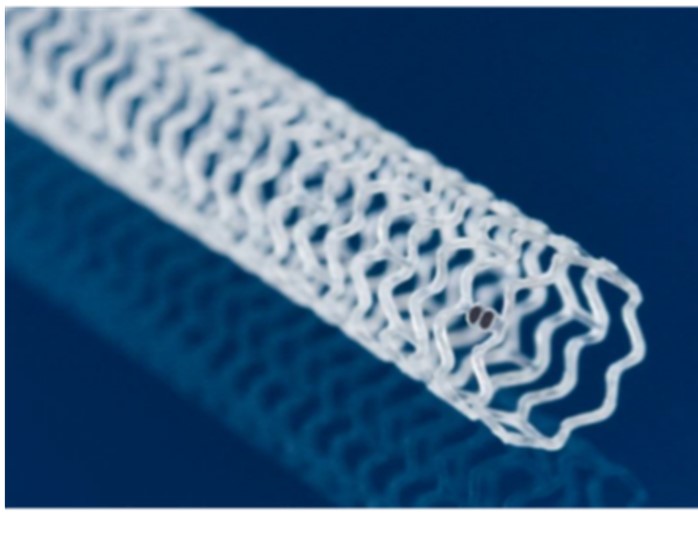
The Esprit BTK [Below-the-Knee] Everolimus Eluting Resorbable Scaffold System (Esprit BTK) is an absorbable, drug-coated mesh tube implanted to widen a narrowed artery in the lower leg to increase low blood flow to the leg and foot (ischemia).
The scaffold is implanted within the lower leg artery to help keep the blood vessel open while the drug is released over time to help prevent the vessel from re-narrowing. To do this, the physician inserts a flexible tube called a catheter with the scaffold mounted on a small balloon at the end into a blood vessel and advances into the affected artery in the lower leg. The catheter is positioned at the narrowed portion of the artery, and the balloon is inflated, which expands the scaffold and presses it against the artery wall. This procedure may be followed by repeat balloon inflations within the scaffold to achieve the desired expansion.
The Esprit BTK is used to treat patients with chronic limb-threatening ischemia, which is associated with rest pain, wounds that do not heal, amputation, and increased mortality. This condition is caused by a buildup fatty substances, such as cholesterol, and calcification that form “plaque” along the lining of the arteries. The plaque formation can reduce, or totally block blood flow to the lower limb, and lead to persistent wounds in the feet.
The scaffold helps keep open the lower leg artery, aided by the gradual release of the everolimus over time. In a study of 261 patients, the device was considered effective in 75% of patients compared to only 44% of patients who received a treatment with no scaffold. Effective treatment was defined as patients who had no amputation above the ankle, no need for an additional procedure, and the artery remained open without significant re-narrowing for 1 year after treatment. Additionally, ~97% of patients had no major safety events (amputation above the ankle or additional procedure through 6 months and no deaths through 30 days).
The Esprit BTK should not be used in patients who are:
- Allergic, hypersensitive to, or otherwise cannot tolerate blood-thinning drugs preventing blood clots administered during or after the procedure.
- Hypersensitive to or otherwise unable to tolerate everolimus, polylactic acid and platinum compounds, or other components in the scaffold.