GORE EXCLUDER Conformable AAA Endoprosthesis (EXCC) - P200030/S014
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval of this panel track supplement.
Product Name: GORE EXCLUDER Conformable AAA Endoprosthesis (EXCC)
PMA Applicant: W.L. Gore & Associates, Inc.
Address: 32360 N. North Valley Parkway, Phoenix, AZ 85085
Approval Date: April 5, 2024
Approval Letter: Approval Order
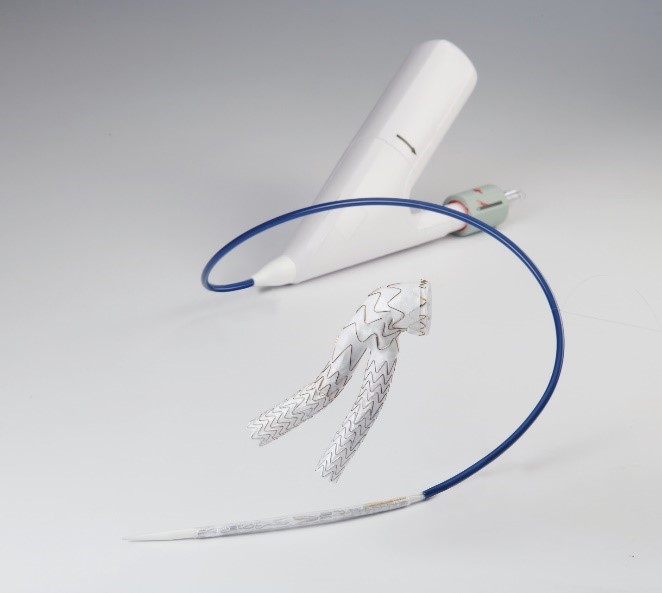
What is it?
The Gore Excluder Conformable AAA Endoprosthesis is a device used to treat a weakened and bulging section (aneurysm) of the infrarenal abdominal aorta, a section of the largest artery in the abdomen located below the renal (kidney) arteries. The multi-component system consists of stent grafts, which are permanent implantable flexible fabric tubes each supported by a metal framework, and delivery catheters, which are long thin tubes used to place the stent grafts.
The delivery catheter containing the stent graft is inserted into the femoral artery (a blood vessel in the upper leg) through a small cut. The delivery system is carefully guided to the site of the aneurysm in the abdominal aorta and placed to provide a new path for blood flow. This relieves the pressure on the aneurysm, which may prevent it from growing or bursting (rupture), which could result in death.
The Gore Excluder Conformable AAA Endoprosthesis is intended for use in patients with an aneurysm of the infrarenal abdominal aorta whose anatomy is adequate to receive the device as defined in the Instructions for Use.
The Gore Excluder Conformable AAA Endoprosthesis may help blood continue to flow while preventing growth or rupture of the aneurysm. In a clinical study of 175 participants, 98.2% were free from a safety event, and 89.7% of study participants were successfully treated without device or aneurysm related complications out to one year after the procedure.
The EXCC should not be used in patients who have:
- Known sensitivities or allergies to the device materials, including expanded polytetrafluoroethylene (ePTFE), fluorinated ethylene propylene (FEP), nickel titanium alloy (Nitinol), and gold.
- A whole-body infection and may be at higher risk of getting an infection from the device.
- Summary of Safety and Effectiveness Data (SSED)
- Labeling – Instructions for Use
- Labeling Part 2 – Patient Information Booklet
- PMA Database Entry