Lumicell Direct Visualization System (DVS) – P230014
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Lumicell Direct Visualization System (DVS)
PMA Applicant: Lumicell, Inc.
Address: Lumicell, Inc., 275 Washington St., Ste. 200, Newton, MA 02458
Approval Date: April 17, 2024
Approval Letter: Approval Order
What is it?
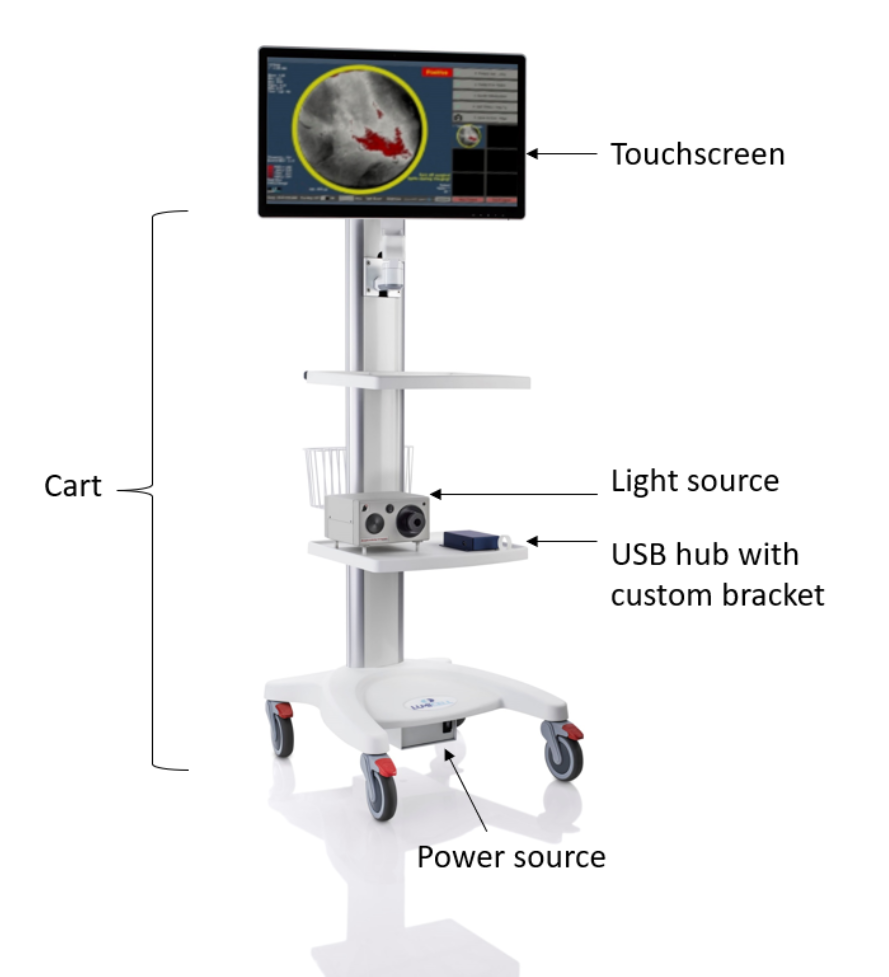
The Lumicell Direct Visualization System (DVS) is a drug and device combination product which provides fluorescence imaging for adjunct breast cancer tissue detection in the surgical cavity after primary specimen removal during lumpectomy surgery. It consists of an optical imaging agent, LUMISIGHT (pegulicianine) (NDA 214511) injected intravenously two to six hours before imaging, and a fluorescence imaging device. The imaging device is used to excite the optical imaging agent, LUMISIGHT, in the cavity, and capture and display real-time fluorescence images that may indicate residual cancer tissue.
During surgery, the handheld probe is used to scan the tumor bed for activated LUMISIGHT by delivering 630 ± 5 nm excitation light and measuring the fluorescence emission signal using a camera after filtering through a 662.5 – 737.5 nm bandpass filter. The resulting data is transferred to the touchscreen and analyzed in real time via Lumicell’s proprietary patient calibrated tumor detection software to highlight regions within the lumpectomy cavity that may contain residual cancer.
The Lumicell DVS is intended to detect remaining cancer cells in the cavity following removal of the primary specimen during lumpectomy surgery in patients with breast cancer.
The device detected residual cancer and guided the removal of the cancerous tissue that would have otherwise remained undetected after Standard of Care in Breast Conserving Surgery in 27 of 357 patients (7.6%.)
Lumicell DVS LUMISIGHT should not be used by patients with a history of hypersensitivity reaction to pegulicianine.
- Summary of Safety and Effectiveness Data (SSED)
- Labeling - Physicians Instructions for Use
- PMA Database Entry