MYNX CONTROL Venous Vascular Closure Device (VCD) 6F-12F – P040044/S097
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: MYNX CONTROL Venous Vascular Closure Device (VCD) 6F-12F
PMA Applicant: Cordis US Corporation
Address: 14201 NW 60th Ave., Miami Lakes, FL 33014
Approval Date: June 27, 2024
Approval Letter: Approval Order
What is it?
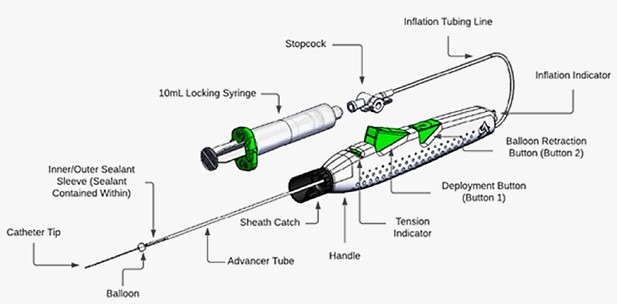
The MYNX CONTROL Venous Vascular Closure Device (VCD) is used to close puncture sites in the large veins of the leg (femoral vein) after a catheterization procedure. Catheterization procedures are used to study or treat a disease or condition in blood vessels or in the heart. The MYNX CONTROL Venous VCD includes gel and a long thin tube (delivery system). The delivery system places the gel into the blood vessel where the catheter was previously inserted.
During catheterization procedures, a small opening (access site) may be made in the femoral vein to insert a catheter, or flexible tube. After the procedure is finished, the catheters are removed. The MYNX CONTROL Venous VCD works by placing a gel made of absorbent chemicals into the access site. When this gel comes into contact with blood it expands and pushes up against the vessel walls. This stops blood from leaking out of the access site.
The MYNX CONTROL Venous VCD is used after catheterization procedures that enter the body through the femoral veins. The device can be used for multiple access sites in the femoral veins.
In a clinical study where the MYNX CONTROL Venous VCD device was used to close more than one access site in a femoral vein, no one (0/100 patients) had a major complication related to the use of the device, such as major bleeding or damage to the vein that needed surgery to be repaired. The average time to stop bleeding at the access site was approximately 2 minutes and 6 seconds and the average time for the patient to regain the ability to walk after the procedure was approximately 2 hours and 36 minutes.
There are no known reasons not to use the MYNX CONTROL Venous VCD.