nAbCyte Anti-AAVRh74var HB-FE Assay – H230005
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Probable Benefit (SSPB) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: nAbCyte Anti-AAVRh74var HB-FE Assay
HDE Applicant: LabCorp Drug Development
Address: 100 Perimeter Park Drive, Ste C, Morrisville, NC 27560
Approval Date: April 25, 2024
Approval Letter: Approval Order
What is it?
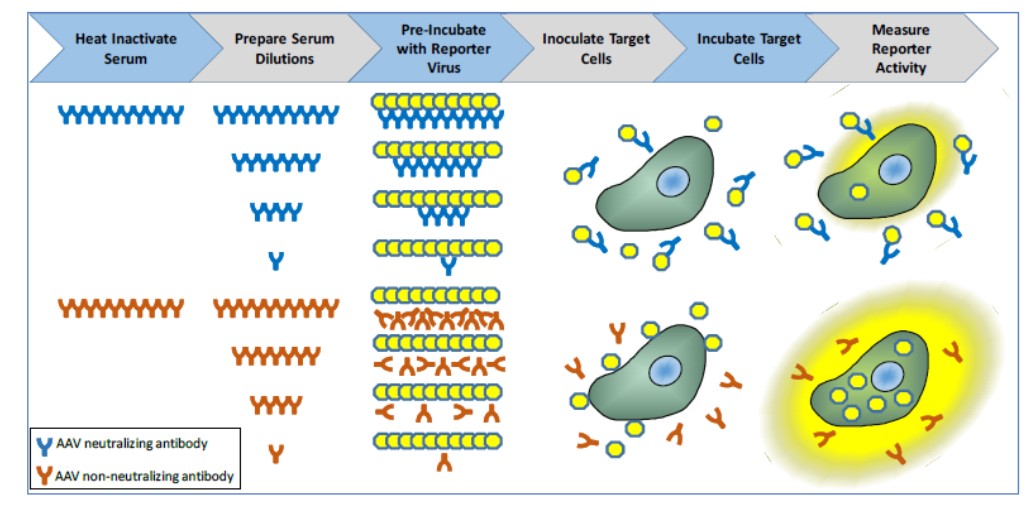
The nAbCyte Anti-AAVRh74var HB-FE Assay is a laboratory test for adults with moderate or severe hemophilia B, a hereditary bleeding disorder caused by a lack of blood clotting factor IX, in which the blood cannot clot properly. This test helps doctors identify who among these patients may benefit from the virus-based gene therapy BEQVEZ (fidanacogene elaparvovec). To be eligible for this treatment, patients must not have been previously infected with adeno-associated virus serotype 74 (AAVRh74var), the virus used in creating the gene therapy. This test detects the presence of neutralizing antibodies to this virus, which indicates if the patient had been previously infected with this virus and is therefore not eligible for this treatment.
A blood sample is taken from a person with moderate or severe hemophilia B and sent to Labcorp-Monogram Biosciences Laboratories for testing. The test uses serum, the clear liquid that remains after blood cells and clotting proteins are removed from the blood sample. The serum is mixed at the laboratory with chemical substances, called reagents, that detect antibodies to the type of adeno-associated virus vector (AAVRh74var) used to deliver the BEQVEZ gene therapy. If the test detects these AAVRh74var antibodies, the person is presumed to be AAVRh74var antibody positive and is not eligible for treatment with BEQVEZ. If the test does not detect these AAVRh74var antibodies, the person is presumed to be AAVRh74var antibody negative and may benefit from treatment with BEQVEZ.
A laboratory professional reviews the results and sends a report to the doctor who ordered the laboratory test. The doctor uses this information to help create a treatment plan for a person who is already diagnosed with moderate or severe hemophilia B.
Doctors use the nAbCyte Anti-AAVRh74var HB-FE Assay to test if a patient with moderate to severe hemophilia B may be eligible for treatment with BEQVEZ.
It will help health care providers who are considering treatment of moderate or severe hemophilia B patients with BEQVEZ determine whether those patients may benefit from treatment with BEQVEZ.
There are no known reasons not to use this test. The clinical data supports the reasonable assurance of safety and probable benefit of this device when used in accordance with the indications for use.