FDA News Release
FDA Proposes Requiring At-a-Glance Nutrition Information on the Front of Packaged Foods
- For Immediate Release:
Español Portuguese French Japanese Korean Traditional Chinese Simplified Chinese Thai
Today, the U.S. Food and Drug Administration is announcing an important step to provide nutrition information to consumers by proposing to require a front-of-package (FOP) nutrition label for most packaged foods. This proposal plays a key role in the agency’s nutrition priorities, which are part of a government-wide effort in combatting the nation’s chronic disease crisis. If finalized, the proposal would give consumers readily visible information about a food’s saturated fat, sodium and added sugars content—three nutrients directly linked with chronic diseases when consumed in excess.
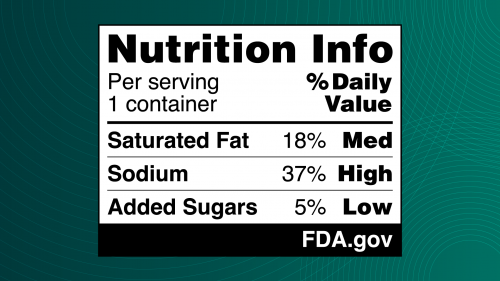
The proposed FOP nutrition label, also referred to as the “Nutrition Info box,” provides information on saturated fat, sodium and added sugars content in a simple format showing whether the food has “Low,” “Med” or “High” levels of these nutrients. It complements the FDA’s iconic Nutrition Facts label, which gives consumers more detailed information about the nutrients in their food.
Chronic diseases, including heart disease, cancer and diabetes, are the leading cause of disability and death in the U.S. With 60% of Americans having at least one chronic disease, such diseases are also the leading drivers of the nation’s $4.5 trillion in annual health care costs. A large body of research indicates that a major contributor to this problem is excess consumption of saturated fat, sodium and added sugars. There is a proliferation of foods in the food supply that are considered ultra processed, which often contain high levels of these nutrients. The Nutrition Info box is focused on providing accessible information to help consumers quickly and easily identify how foods can be part of a healthy diet.
“The science on saturated fat, sodium and added sugars is clear,” said FDA Commissioner Robert M. Califf, M.D. “Nearly everyone knows or cares for someone with a chronic disease that is due, in part, to the food we eat. It is time we make it easier for consumers to glance, grab and go. Adding front-of-package nutrition labeling to most packaged foods would do that. We are fully committed to pulling all the levers available to the FDA to make nutrition information readily accessible as part of our efforts to promote public health.”
The proposed Nutrition Info box is informed by a substantial body of research conducted by the FDA, including a scientific literature review, consumer focus groups and a peer-reviewed experimental study. In 2023, the FDA conducted an experimental study of nearly 10,000 U.S. adults to further explore consumer responses to three different types of FOP labels. The purpose of the experimental study was to identify which FOP schemes enabled participants to make quicker and more accurate assessments of the healthfulness of a product based on the levels of saturated fat, sodium and added sugars displayed. The experimental study showed that the black and white Nutrition Info scheme with the percent Daily Value performed best in helping consumers identify healthier food options.
“Food should be a vehicle for wellness, not a contributor of chronic disease,” said FDA Deputy Commissioner for Human Foods Jim Jones. “In addition to our goal of providing information to consumers, it’s possible we’ll see manufacturers reformulate products to be healthier in response to front-of-package nutrition labeling. Together, we hope the FDA’s efforts, alongside those of our federal partners, will start stemming the tide of the chronic disease crisis in our country.”
The proposed Nutrition Info box is part of the White House National Strategy on Hunger, Nutrition and Health to reduce diet-related diseases by 2030. The Nutrition Info box, the recently updated “healthy” claim, the FDA’s work to develop a “healthy” symbol and the draft Phase II voluntary sodium reduction targets are key aspects of a government-wide approach to improving nutrition and reducing chronic diseases in the U.S. These efforts can help consumers more easily identify foods recommended by the Dietary Guidelines for Americans and may assist them in reducing their consumption of certain nutrients that can be found in foods that are commonly considered ultra-processed. The FDA is committed to continuing its comprehensive, science-based activities to create a healthier food supply, empower consumers with information and support lifelong healthy eating patterns.
The proposed rule, if finalized, would require food manufacturers to add a Nutrition Info box to most packaged food products three years after the final rule’s effective date for businesses with $10 million or more in annual food sales and four years after the final rule’s effective date for businesses with less than $10 million in annual food sales.
Comments on the proposed rule can be submitted electronically to http://www.regulations.gov by May 16, 2025.
Related Information
###
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, radiation-emitting electronic products, and for regulating tobacco products.
Inquiries
- Media:
- Enrico Dinges
- 240-620-9293
- Consumer:
- 888-INFO-FDA
