COMPANY ANNOUNCEMENT
Endo Expands Voluntary Recall of Clonazepam Orally Disintegrating Tablets, USP (C-IV) Due to Potential Product Carton Strength Mislabeling
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionMislabeled with the incorrect strength on the carton
- Company Name:
- Endo, Inc.
- Brand Name:
-
Brand Name(s)Par Pharmaceutical
- Product Description:
-
Product DescriptionClonazepam Orally Disintegrating Tablets, USP (C-IV)
Company Announcement
MALVERN, PA, November 18, 2024 – Endo, Inc. (OTCQX: NDOI) (“Endo”) announced today that one of its operating subsidiaries, Endo USA, Inc., is expanding its previously announced voluntary recall of Clonazepam Orally Disintegrating Tablets, USP (C-IV) due to potential product carton strength mislabeling.
Specifically, Endo’s ongoing investigation has identified the possibility that the Clonazepam product lots listed below contain a limited number of cartons printed with the incorrect strength and National Drug Code (NDC) code due to an error by a third-party packager. The blister strips and tablets inside the product pack reflect the correct strength for the lot.
The following table details the lots being added to the voluntary recall, including lot product description and NDC number.
Potential Product Description / NDC Number | Lot # | Expiry date |
|---|---|---|
Clonazepam ODT, USP (C-IV) 2mg / 49884-310-02 | 550176501 | Feb 2027 |
550176601 | Feb 2027 | |
Clonazepam ODT, USP (C-IV) 0.125mg / 49884-306-02 | 550174101 | Jan 2027 |
Clonazepam ODT, USP (C-IV) 0.25mg / 49884-307-02 | 550142801 | Aug 2026 |
550142901 | Aug 2026 | |
550143001 | Aug 2026 | |
550143101 | Aug 2026 | |
550143201 | Aug 2026 | |
550143301 | Aug 2026 | |
550143401 | Aug 2026 | |
550147201 | Aug 2026 | |
550147401 | Aug 2026 | |
Clonazepam ODT, USP (C-IV) 1mg / 49884-309-02 | 550145201 | Aug 2026 |
550175901 | Feb 2027 | |
550176001 | Feb 2027 | |
550176201 | Feb 2027 |
Risk Statement:
Children and adults who inadvertently consume a higher dose of clonazepam could be at increased risk for the adverse events of significant sedation, confusion, dizziness, diminished reflexes, ataxia, and hypotonia. There is reasonable probability for significant, possibly life-threatening, respiratory depression especially for patients with concomitant pulmonary disease, patients who have prescribed dosing near maximal dosing, and patients also taking other medications that could cause additional respiratory depression.
To date, Endo has not received any reports of adverse events associated with this product recall.
Clonazepam Orally Disintegrating Tablets are indicated alone or as an adjunct in the treatment of the Lennoz-Gastaut syndrome (petit mal variant), akinetic and myoclonic seizures. Additionally, the product is indicated for the treatment of panic disorder.
Package Identification:
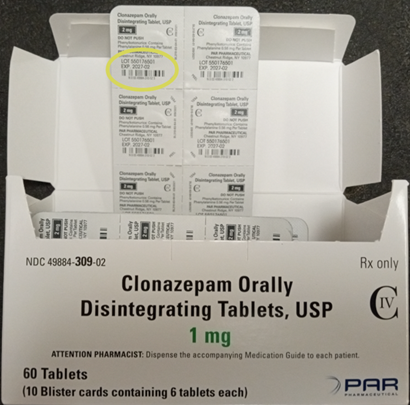
The product is packaged in cartons containing 60 tablets packed into 10 blister strips each containing 6 tablets. The carton and each blister strip pocket are printed with the name, strength, lot number, expiration date, and NDC number. The packaging lists the legacy company Par Pharmaceutical which previously marketed clonazepam before the product was acquired by Endo.
The images below provide an example of the potential mislabeling showing the components of a package of Clonazepam Orally Disintegrating tablets, USP 2 mg lot 550176501 with a carton bearing the product description and NDC code of Clonazepam Orally Disintegrating Tablets, USP 1 mg 60-count. The location of the lot number on each component of the package is shown on the photographs below.
Action Required:
The product lots were distributed through wholesale distributors to retail pharmacies nationwide.
Endo is providing written notification to wholesale accounts and retailers that have received the product lots and is arranging for the return of all existing inventory through Inmar, Inc.
Distributors, retailers that have the product lot being recalled should immediately stop distributing and dispensing and return to the place of purchase or contact Inmar on the below telephone line.
Consumers in possession of any unused prescribed tablet cartons of Clonazepam Orally Disintegrating tablets, USP bearing the above lot numbers have been advised to discontinue use of the product.
In the event that a patient inadvertently took an incorrect dose rather than the intended dose, they are advised to consult a physician.
Consumers with questions regarding this recall can contact Inmar by telephone at 877-890-0765 (Monday through Friday, 9 a.m. to 5 p.m. ET) or by email at rxrecalls@inmar.com.
For more information about Clonazepam Orally Disintegrating Tablets, USP, please see full Prescribing Information including BOXED WARNING available at DailyMed - CLONAZEPAM tablet, orally disintegrating (nih.gov).
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About Endo
Endo is a diversified pharmaceutical company boldly transforming insights into life- enhancing therapies. Our passionate team members collaborate to develop and deliver these essential medicines. Together, we are committed to helping everyone we serve live their best life. Learn more at www.endo.com or connect with us on LinkedIn.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements including but not limited to any statements related to product recalls, mislabeling, adverse events, FDA or other regulatory actions and any other statements that refer to expected, estimated or anticipated future results or that do not relate solely to historical facts. Statements including words such as "believes," "expects," "anticipates," "intends," "estimates," "plan," "will," "may," "look forward," "guidance," "future," "potential" or similar expressions are forward-looking statements. Because these statements reflect Endo's current views, expectations and beliefs concerning future events, they involve risks and uncertainties, some of which Endo may not currently be able to predict. Although Endo believes that these forward-looking statements and other information are based upon reasonable assumptions and expectations, readers should not place undue reliance on these or any other forward-looking statements and information. Actual results may differ materially and adversely from current expectations based on a number of risks, uncertainties and factors, including risks and uncertainties related to the recall and any future recalls, potential adverse events and any regulatory actions by the FDA. Endo assumes no obligation to publicly update any forward- looking statements, whether as a result of new information, future developments or otherwise, except as may be required under applicable securities laws. Additional information concerning risk factors, including those referenced above, can be found in press releases issued by Endo and in Endo's public filings with the U.S. Securities and Exchange Commission, including the discussion under the heading "Risk Factors" in Endo's most recent Form 10-Q and in Endo's final prospectus filed pursuant to Rule 424(b) under the Securities Act of 1933, as amended, in connection with its Form S-1/A.
Media: Investors:
Linda Huss Juan Avendano
media.relations@endo.com investor.relations@endo.com
Company Contact Information
- Consumers:
- Inmar, Inc.
- 877-890-0765
- Media:
- Linda Huss
- media.relations@endo.com