COMPANY ANNOUNCEMENT
IBSA Pharma Inc. Issues Voluntary Nationwide Recall of Select Lots of TIROSINT®-SOL (levothyroxine sodium) Oral Solution Due to Subpotency
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionSubpotency
- Company Name:
- IBSA Pharma Inc.
- Brand Name:
-
Brand Name(s)IBSA
- Product Description:
-
Product DescriptionTIROSINT®-SOL (levothyroxine sodium)
Company Announcement
IBSA Pharma Inc. is voluntarily recalling 27 lots of TIROSINT®-SOL (levothyroxine sodium) Oral Solution to the consumer level. This voluntary recall has been initiated because these lots may be subpotent. The company’s analyses show a slight decrease below 95.0% of its labeled amount in levothyroxine sodium (T4) for some lots.
This recall does not apply to TIROSINT® (levothyroxine sodium) capsules.
Risk Statement: Patients being treated for hypothyroidism (underactive thyroid), who receive subpotent TIROSINT®-SOL, may experience signs and symptoms of hypothyroidism (underactive thyroid) which may include, fatigue, increased sensitivity to cold, constipation, dry skin, puffy face, hair loss, slow heart rate, depression, swelling of the thyroid gland and/or unexplained weight gain or difficulty losing weight. Over- or under-treatment with TIROSINT® SOL may have negative effects on growth and development, cardiovascular function, bone metabolism, reproductive function, cognitive function, emotional state, gastrointestinal function, and glucose and lipid metabolism. Any patients including those who might be pregnant, newborn infants, or elderly patients, should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product. To date, IBSA Pharma Inc. has not received any reports of adverse events that have been determined to be related to this voluntary recall.
TIROSINT®-SOL is indicated for:
- Hypothyroidism - As replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism.
- Pituitary Thyrotropin (Thyroid-Stimulating Hormone, TSH) Suppression - As an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent well differentiated thyroid cancer.
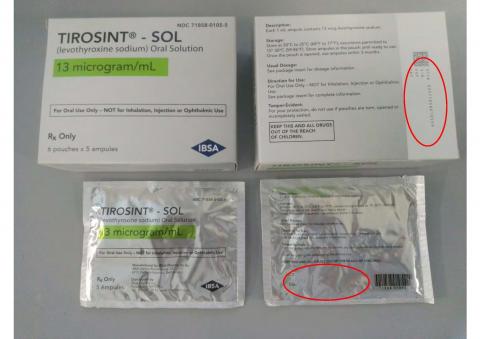
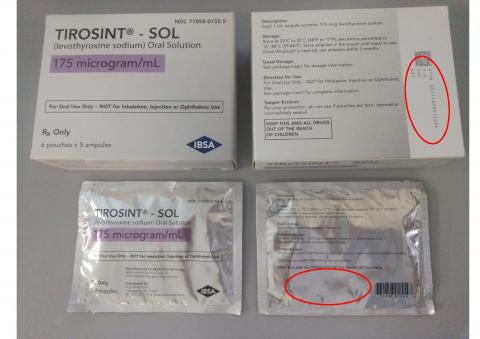
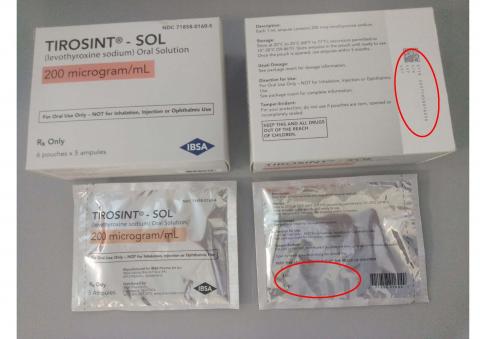
TIROSINT®-SOL oral solution is a clear, colorless to slightly yellow solution supplied in a 1 mL white, non-transparent, unit-dose ampule. The dosage strength is identified on the box and the pouch, and is associated with a distinct color. Each ampule bears a colored label with the dosage strength and the product name (TIROSINT®-SOL). For full prescribing information, visit www.TirosintSOL.com.
The product description, NDC numbers, lot numbers, and expiration dates of affected TIROSINT® SOL lots are shown in the table below, and photos of the products can be found at the end of this press release.
| Product Description | NDC | Lot Number | Expiration Date |
|---|---|---|---|
| TIROSINT-SOL 13 mcg/mL 30 units carton-box | 71858-0105-5 | 220409 | 10/2023 |
| TIROSINT-SOL 13 mcg/mL 30 units carton-box | 71858-0105-5 | 220956 | 03/2024 |
| TIROSINT-SOL 25 mcg/mL 30 units carton-box | 71858-0110-5 | 220856 | 02/2024 |
| TIROSINT-SOL 37.5 mcg/mL 30 units carton-box | 71858-0112-5 | 220552 | 11/2023 |
| TIROSINT-SOL 37.5 mcg/mL 30 units carton-box | 71858-0112-5 | 221055 | 04/2024 |
| TIROSINT-SOL 44 mcg/mL 30 units carton-box | 71858-0113-5 | 220553 | 11/2023 |
| TIROSINT-SOL 44 mcg/mL 30 units carton-box | 71858-0113-5 | 221056 | 04/2024 |
| TIROSINT-SOL 50 mcg/mL 30 units carton-box | 71858-0115-5 | 220407 | 10/2023 |
| TIROSINT-SOL 50 mcg/mL 30 units carton-box | 71858-0115-5 | 220960 | 03/2024 |
| TIROSINT-SOL 62.5 mcg/mL 30 units carton-box | 71858-0117-5 | 220556 | 11/2023 |
| TIROSINT-SOL 62.5 mcg/mL 30 units carton-box | 71858-0117-5 | 221058 | 04/2024 |
| TIROSINT-SOL 75 mcg/mL 30 units carton-box | 71858-0120-5 | 220853 | 02/2024 |
| TIROSINT-SOL 88 mcg/mL 30 units carton-box | 71858-0125-5 | 220411 | 10/2023 |
| TIROSINT-SOL 88 mcg/mL 30 units carton-box | 71858-0125-5 | 220854 | 02/2024 |
| TIROSINT-SOL 100 mcg/mL 30 units carton-box | 71858-0130-5 | 220413 | 10/2023 |
| TIROSINT-SOL 100 mcg/mL 30 units carton-box | 71858-0130-5 | 220964 | 03/2024 |
| TIROSINT-SOL 112 mcg/mL 30 units carton-box | 71858-0135-5 | 220414 | 10/2023 |
| TIROSINT-SOL 112 mcg/mL 30 units carton-box | 71858-0135-5 | 220852 | 02/2024 |
| TIROSINT-SOL 112 mcg/mL 30 units carton-box | 71858-0135-5 | 220970 | 03/2024 |
| TIROSINT-SOL 125 mcg/mL 30 units carton-box | 71858-0140-5 | 220855 | 02/2024 |
| TIROSINT-SOL 137 mcg/mL 30 units carton-box | 71858-0145-5 | 220415 | 10/2023 |
| TIROSINT-SOL 137 mcg/mL 30 units carton-box | 71858-0145-5 | 221052 | 04/2024 |
| TIROSINT-SOL 150 mcg/mL 30 units carton-box | 71858-0150-5 | 220959 | 03/2024 |
| TIROSINT-SOL 175 mcg/mL 30 units carton-box | 71858-0155-5 | 220416 | 10/2023 |
| TIROSINT-SOL 175 mcg/mL 30 units carton-box | 71858-0155-5 | 221053 | 04/2024 |
| TIROSINT-SOL 200 mcg/mL 30 units carton-box | 71858-0160-5 | 220418 | 10/2023 |
| TIROSINT-SOL 200 mcg/mL 30 units carton-box | 71858-0160-5 | 220560 | 11/2023 |
IBSA Pharma Inc. is proactively notifying its wholesalers, retailers and healthcare providers to discontinue distribution of the product being recalled and is arranging for the return of all recalled products. Patients who are currently taking TIROSINT®-SOL should not discontinue use without contacting their healthcare provider for further guidance and/or replacement prescription.
Consumers and healthcare providers with questions regarding this recall can contact IBSA Pharma Inc. by phone number at 1-800-587-3513 Monday through Friday between the hours of 9:00 am to 7:00 pm (EST), or by e-mail at medinfo@ibsapharma.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Wholesalers and retailers with questions regarding this recall can contact IBSA Pharma Inc. by phone number 855-224-0231 Monday through Friday between the hours of 8:00 am to 5:00 pm (CST), or by e-mail at IBSACS@Eversana.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.