COMPANY ANNOUNCEMENT
MyNicNaxs, LLC Issues Voluntary Nationwide Recall of Various Dietary Supplements Due to Undeclared Active Pharmaceutical Ingredient (API)
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Dietary Supplements
- Reason for Announcement:
-
Recall Reason DescriptionUndeclared active pharmaceutical ingredients (API)
- Company Name:
- MyNicNaxs, LLC
- Brand Name:
-
Brand Name(s)Platinum, Slimming Plus and more
- Product Description:
-
Product Descriptiondietary supplements
Company Announcement
MyNicNaxs, LLC, Deltona, FL is voluntarily recalling all lots of dietary supplements distributed nationwide to the consumer level. The products have been found to contain undeclared active pharmaceutical ingredients (API)The presence of Sildenafil, Sibutramine, Diclofenac and/or Phenolphthalein in the dietary supplements renders it an unapproved drug for which safety and efficacy have not been established and, therefore, subject to recall. These products were distributed from January 2013, to December 2017, though our website http://www.mynicnaxs.com. The undeclared drug ingredients found in these products may pose serious health risks because consumers with underlying medical issues may take them without knowing that they can cause serious harm or interact in dangerous ways with other drugs they may be taking. API found in FDA samples include the following:
Sibutramine is the active pharmaceutical ingredient in Meridia, a new drug approved by FDA for marketing in 1997 for prescription treatment of obesity and, subsequently, withdrawn from the U.S. market on December 21, 2010, after clinical data indicated Sibutramine poses an increased risk of heart attack and stroke.
Phenolphthalein is a known carcinogen (cancer causing agent) that was once an ingredient used in over-the-counter laxatives but is no longer approved for marketing in the United States.
Sildenafil is the active pharmaceutical ingredient in Viagra (PDE-5 inhibitor), a drug approved by FDA for the treatment of erectile dysfunction. PDE-5 inhibitors may interact with nitrates found in some prescription drugs (such as nitroglycerin) and can lower blood pressure to dangerous levels. Consumers with diabetes, high blood pressure, high cholesterol, or heart disease often take nitrates.
Diclofenac is a non-steroidal anti-inflammatory drugs (NSAIDs) found in FDA-approved drugs that are used to treat pain and inflammation associated with several conditions. NSAIDs could lead to serious gastrointestinal (GI) adverse events such as bleeding, ulceration, and fatal perforation of the stomach and intestines. Patients who are already taking medication that could cause bleeding may increase their risk for bleeding significantly.
Products listed below are being voluntarily recalled due to undeclared active pharmaceutical ingredients. The products can be identified by referring to the table below:
| Product Description | API Found in FDA Lab Results |
|---|---|
| Platinum Maximum Strength Blue Pill Version; 30 capsules; 500mg each |
Sibutramine and Phenolphthalein |
| Platinum Maximum Strength Blue Pill Version; 30 capsules; 500mg each |
Sibutramine and Phenolphthalein |
| Slimming Plus Advanced Weight Loss; 30 capsules; 500mg each |
Sibutramine and Phenolphthalein |
| African Viagra - sexual performance enhancement product; 4500mg x 2 |
Sildenafil |
| GINSENG - sexual performance enhancement product; 300mg/tablet x 10 tablets |
Sildenafi |
| African Superman - sexual performance enhancement product; 2900mg x 8 tablets per blister pack |
Sildenafil |
| Old Chinese - sexual performance enhancement product; 19800mg x 10 capsules |
Sildenafil |
| Lean Extreme Max; 30 capsules; 400mg each | Sibutramine |
| X-treme Beauty Slim; 30 capsules; 350mg each | Sibutramine |
| African Superman - Top-Class Permanence Tablet; 2900mg x 8 tablets |
Sildenafil |
| Slim Evolution - 100% Natural Ingredients; 30 capsules; 350mg each |
Diclofenac |
| Meizitang Strong Version capsules packed in a non-flexible clear bottle with a green screw-on top |
Sibutramine |
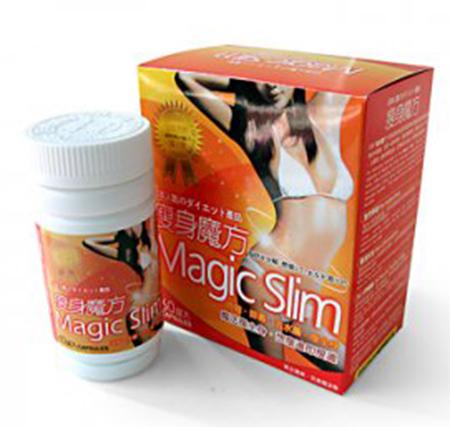
| Magic Slim capsules packed in a non-flexible white bottle with a white screw-on top |
Sibutramine |
| Slim Xtreme capsules packed in a non-flexible white bottle with a white screw-on top |
Sibutramine |
| Meizi Evolution capsules were packed in a non- flexible clear bottle with a blue screw-on top |
Sibutramine |
| SlimEasy Herbs capsules packed in blister packaging and placed in a white box with black labeling |
Sibutramine |
| Hokkaido - capsules packed in blister packaging in pink box with black labeling |
Phenolphthalein |
| Super Fat Burning Bomb capsules in blister packs, packaged in a red box with black labeling |
Sibutramine and Phenolphthalein |
| FRUTA Bio blister packs, packaged in a yellow/green box with green labeling |
Sibutramine and Phenolphthalein |
| JIANFEIJINDAN Activity Girl - blister packs, packaged in a white/pink box with pink labeling |
Sibutramine |
| Reduce Weight FRUTA PLANTA blister packs, packaged in a yellow/green box with green labeling |
Phenolphthalein |
| Fat Loss Slimming Beauty – 30 capsules in blister packs packaged in yellow/black box -500 mg |
Sibutramine and Phenolphthalein |
| Fruta Planta -blister packs packaged in yellow/green box with green labeling |
Sibutramine and Phenolphthalein |
| Botanical Slimming - 100% Natural Soft gel; 30 soft gels; 650mg each packaged in a green bag with yellow and white lettering | |
| Slim Body - Dietary Supplement;100% Herbal Slimming Formula; 30 capsules; 6x5x300mg blister packs, packaged in blue and red box | |
MyNicNaxs LLC, is notifying its distributors and customers by e-mail and is arranging for return of all recalled products. No reports of adverse events have been reported to date.
Consumers are advised to not consume and discontinue use of the products immediately. For any questions regarding this recall, contact Mike Banner by phone 407-791-3597 or Chevonne Torres 386-337-8142, Monday to Friday, 09:00am-5:00 pm, Eastern Time.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Mike Banner, Chevonne Torres
- 386-337-8142