COMPANY ANNOUNCEMENT
Sun Pharmaceutical Industries, Inc. Issues Voluntary Nationwide Recall of Vecuronium Bromide for Injection Due to the Presence of Particulate Matter Identified as Glass
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
Generic Drugs - Reason for Announcement:
-
Recall Reason DescriptionPotential Foreign Material
- Company Name:
- Sun Pharmaceutical Industries, Inc
- Brand Name:
-
Brand Name(s)Sun Pharma
- Product Description:
-
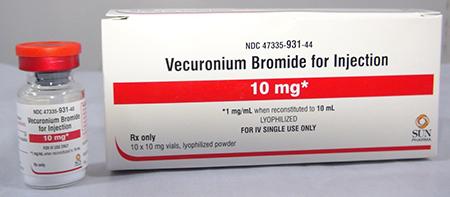
Product DescriptionVecuronium Bromide for Injection
Company Announcement
Sun Pharmaceutical Industries, Inc. (SPII), a wholly owned subsidiary of Sun Pharmaceutical Industries, Ltd. is voluntarily recalling three lots of Vecuronium Bromide for Injection, 10 mg (lyophilized powder), and one lot of Vecuronium Bromide for Injection, 20 mg (lyophilized powder) to the hospital level. The Vecuronium Bromide for Injection has been found to contain particulate matter identified as glass.
The administration of a glass particulate, if present in an intravenous drug, may result in local irritation or swelling in response to the foreign material. More serious potential outcomes would include blockage and clotting in blood vessels, which may be life-threatening. To date, SPII has not received any reports of adverse events related to this recall.
Vecuronium Bromide for Injection is used as an adjunct to general anesthesia, to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation and is packaged in a glass vial; ten vials per carton. Vecuronium Bromide for Injection should be administered by or under the supervision of experienced clinicians and must be reconstituted prior to use. The affected Vecuronium Bromide for Injection, include the following:
| Product Name | Lot Number | NDC Number | Expiration Date | Number of Units |
|---|---|---|---|---|
| Vecuronium Bromide for Injection, 20 mg | JKS0400A | 47335-932-44 [carton] 47335-932-40 [vial] |
03/2019 | 1384 cartons |
| Vecuronium Bromide for Injection, 10 mg | JKS0443A | 47335-931-44 [carton] 47335-931-40 [vial] |
03/2019 | 4404 cartons |
| Vecuronium Bromide for Injection, 10 mg | JKS0444A | 47335-931-44 [carton] 47335-931-40 [vial] |
03/2019 | 3744 cartons |
| Vecuronium Bromide for Injection, 10 mg | JKS0477A | 47335-931-44 [carton] 47335-931-40 [vial] |
03/2019 | 4386 cartons |
The product can be identified by vial or carton labeled as Vecuronium Bromide for Injection containing the specific Lot Number and Expiration Dates mentioned above.
This product was distributed nationwide to wholesale customers and medical facilities.
On January 3, 2019, SPII notified its distributors and customers through its 3rd party, Recall Coordinator (Inmar Inc.), via FedEx standard overnight shipping and has arranged for return via prepaid FedEx Ground shipping of all recalled products.
Distributors and medical facilities that have Vecuronium Bromide for Injection, which is being recalled, should stop using and return it to place of purchase or as directed in the recall notification.
Consumers with questions regarding this recall can contact SPII by calling 1-800-406-7984 Monday through Friday between 8:00 am to 5:00 pm EST or e-mailing drug.safetyUSA@sunpharma.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems associated with the use of this product may be reported to FDA's MedWatch Adverse Event Reporting program either by phone, on line, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- SPII
- 1-800-406-7984
- Drug.safetyUSA@sunpharma.com
- Media:
- Gaurav Chugh
- 91 22 4324 4324 x 5373
- gaurav.chugh@sunpharma.com