COMPANY ANNOUNCEMENT
Windstone Medical Packaging dba Aligned Medical Solutions Issues Nationwide Recall of Cardinal Health’s Monoject™ Flush Prefilled Saline Syringes in Kits Due to Plunger Defect
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Medical Devices
- Reason for Announcement:
-
Recall Reason DescriptionPotential for the plunger to draw back after the air has been expelled and reintroduced air back into the syringe
- Company Name:
- Windstone Medical Packaging dba Aligned Medical Solutions
- Brand Name:
-
Brand Name(s)Cardinal Health’s Monoject™
- Product Description:
-
Product DescriptionFlush Prefilled Saline Syringes
Company Announcement
On August 19, 2021, Aligned Medical Solutions initiated a nationwide recall of Cardinal Health’s Monoject™ Flush Prefilled Saline Syringes placed into 9,378 kits. Including;
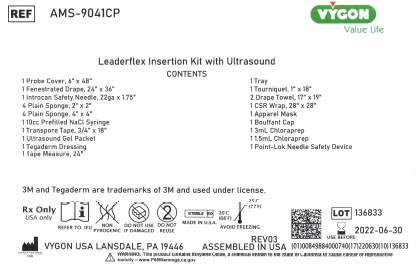
- 1 lot of AMS-9041CP Leaderflex Insertion Kit with Ultrasound
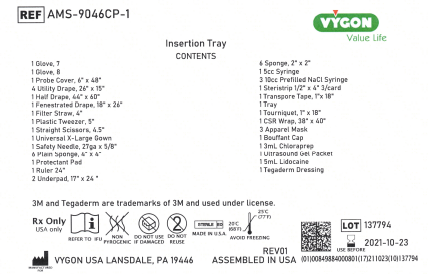
- 1 lot of AMS-9046CP-1 Insertion Tray-RX
- 45 lots of AMS8939A Universal Procedure Pack w/Split Drape
- 1 lot of AMS9957A Port Insertion Pack
- 3 lots of AMS12149 Procedure Pack
These convenience kits have been found to contain the Cardinal Health’s Monoject™ Flush Prefilled Saline Syringe part # 8881570121, which has been recalled for the potential for the plunger to draw back after the air has been expelled and reintroduced air back into the syringe. If a clinician is not aware of air being introduced into the syringe, the clinician could inadvertently push air into the vascular system creating the potential for an air embolism. Injection of air into the vascular system can cause air embolism which can result in serious adverse health consequences or death.
See Cardinal Health’s Urgent Medical Device Recall Event 2021-04063 Monoject™ Flush Prefilled Saline Syringes https://www.cardinalhealth.com/en/cmp/ext/corp/monoject-flush-prefilled-saline-syringes-issue.html
Consumers who have affected product(s) should immediately review their inventory and quarantine all affected kits. Contact the Quality Department for further instructions on labeling and replacement product if needed at 407-638-9924. Customers will be provided with a yellow label that is to be placed on the packs containing the recalled Cardinal Health’s Monoject™ Flush Prefilled Saline Syringe. The label will read as follows;
- At the time the kit is opened for use the prefilled syringe manufactured by Cardinal Health should be identified, set aside and not used. Do not use the prefilled syringe in the kit.
- The recalled prefilled syringe should be rendered unusable to protect against inadvertent use and disposed or pursuant to the medical waste policies in effect at your institution
Consumers can request replacement syringes by calling 407-638-9924.
Recalled Product(s) were manufactured from 01/13/2020 to 10/14/2020 and distributed from 01/23/2020 to 10/19/2020.
The following kit part numbers and lot numbers containing the recalled /affected Cardinal Health Monoject™ Flush Prefilled Syringes have been recalled:
| Part Number | Description | Lot # |
|---|---|---|
| AMS-9041CP | Leaderflex Insertion Kit with Ultrasound | 136833 |
| AMS-9046CP-1 | Insertion Tray-RX | 137794 |
| AMS8939A | Universal Procedure Pack w/Split Drape | 140390, 141087, 141088, 141096, 141097, 141098, 141100, 141101, 141102, 141717, 145615, 146037, 146039, 146349, 146351, 146766, 146768, 146905, 146908, 147902, 147903, 148409, 148410, 148411, 148412, 148413, 148414, 148450, 148451, 148452, 148636, 148638, 148639, 148640, 149131, 149132, 149133, 149134, 149135, 149136, 149137, 149138, 149484, 149485, 149486 |
| AMS9957A | Port Insertion Pack | 147773 |
| AMS12149 | Procedure Pack | 145718, 146897, 148612 |
Product(s) can be identified by the pack label inside the sterile barrier of the convenience kit.
Aligned Medical Solutions has notified the FDA of this action.
No injuries have been reported to date.
Aligned Medical Solutions is notifying its distributors by email and will arrange for labels to identify the packs with the recalled syringe. Aligned Medical Solutions will also arrange for replacement syringes to be sent for all recalled product(s).
Aligned Medical Solutions distributed these packs Nationwide.
Consumers with questions may contact the company via telephone at 1-800 -637-7056 between the hours of 8:00 am and 5:00 pm (Mountain Time). Consumer may also contact the company via e-mail at fieldcorrectiveaction@alignedmedicalsolutions.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Windstone Medical Packaging dba Aligned Medical Solutions
- 1-800 -637-7056
- fieldcorrectiveaction@alignedmedicalsolutions.com