FDA's HSP/BIMO Initiative Accomplishments: Update September 2010
Printer-Friendly Version of Report (PDF - 98 KB)
In the 18 months since issuance of the first progress report, the Food and Drug Administration has continued the Human Subject Protection (HSP)/Bioresearch Monitoring (BIMO) Initiative (1), (2) intended to modernize and strengthen the agency's oversight and protection of subjects in clinical trials and the integrity of resulting data. The HSP/BIMO Initiative encompasses all FDA-regulated clinical trials, that is, those related to human drugs and biological drug products, devices, foods, and veterinary medicine. The overarching goals of the agency's BIMO program are to protect the rights, safety, and welfare of subjects involved in FDA-regulated clinical trials; to determine the accuracy and reliability of clinical trial data submitted to FDA in support of research or marketing applications; and to assess compliance with FDA's regulations governing the conduct of clinical trials, including those for informed consent and ethical review. Below are significant accomplishments and initiatives from January 2009 through the present.
- New Regulations
- New Guidance
- Improvements to FDA's Internal Procedures
- Clinical Trial Transformation Initiative (CTTI)
- International Harmonization, Capacity-Building, and Outreach Activities
- Activities in Progress
- Guidance in Development
- Modernizing FDA Regulations
- BIMO Inspections for Fiscal Year 2009
- Footnotes
- Registration of Institutional Review Boards (IRBs) – Final Rule.(3) This new regulation, which requires IRBs to register through a system maintained by the Office for Human Research Protections, will make it easier for FDA to identify IRBs that review FDA-regulated research and convey educational information to them. (January 2009)
- Expanded Access to Investigational Drugs for Treatment Use – Final Rule.(4) This revision clarifies existing regulations and adds new types of expanded access for treatment use. It is intended to improve access to investigational drugs for patients with serious or immediately life-threatening diseases or conditions who lack other therapeutic options and who may benefit from such therapies. (August 2009)
- Charging for Investigational Drugs Under an Investigational New Drug Application – Final Rule.(5) This new regulation permits sponsors to charge for a broader range of investigational and expanded access uses. It clarifies the specific circumstances and the types of costs for which a manufacturer can charge patients when an investigational new drug is used as part of a clinical trial or when it is used outside the scope of a clinical trial. (August 2009)
- Informed Consent Elements - Proposed Rule.(6) The Food and Drug Administration Amendments Act of 2007 (FDAAA) requires that FDA update its informed consent regulations to require that the informed consent documents and processes for certain clinical investigations include a statement that clinical trial information for such investigations has been or will be submitted for inclusion in the National Institutes of Health/National Library of Medicine clinical trial registry databank. The proposed rule addresses this new requirement for informed consent. (December 2009)
- Reporting Information Regarding Falsification of Data - Proposed Rule.(7) FDA proposed to amend its regulations to require sponsors to report information indicating that any person has, or may have, engaged in the falsification of data in the course of reporting study results, or in the course of proposing, designing, performing, recording, supervising, or reviewing studies that involve human subjects or animal subjects conducted by or on behalf of a sponsor or relied on by a sponsor. FDA proposed this change because ambiguity in the current reporting scheme has caused confusion among sponsors. The proposed rule is intended to help ensure the validity of data that the agency receives in support of applications and petitions for FDA product approvals and authorization of certain labeling claims and to protect research subjects. (February 2010)
- IRB Registration - Frequently Asked Questions – Final Guidance.(8) This guidance is intended to assist IRBs in complying with the new requirement for IRB registration (21 CFR 56.106), which became effective September 14, 2009. The guidance addresses basic information, such as why FDA issued the new rule, which IRBs are subject to the new regulation, the type of information to be provided when registering, and implications of non-compliance. (July 2009)
- Investigator Responsibilities – Protecting the Rights, Safety, and Welfare of Study Subjects – Final Guidance.(9) This guidance is intended to help investigators who conduct FDA-regulated research better meet their responsibilities with respect to protecting human subjects and ensuring the integrity of the data from clinical investigations. The guidance clarifies FDA’s expectations concerning the investigator’s responsibility to (1) supervise a clinical study in which some study tasks are delegated to employees or colleagues of the investigator or other third parties, and (2) protect the rights, safety, and welfare of study subjects. (October 2009)
- Statement of Investigator - Frequently Asked Questions – Final Guidance.(10) FDA developed this guidance in response to numerous questions from the research community regarding the Form FDA 1572. In this guidance, FDA provides answers to frequently asked questions concerning the purpose of the form and the information to be provided. The guidance also clarifies when the form should be completed and signed by the investigator, particularly for investigators participating in studies conducted outside the United States that may or may not be under an investigational new drug (IND) application. (June 2010)
- Information Sheet Guidances on Clinical Investigator Disqualification (11) and FDA Inspections of Clinical Investigators(12) – Final Guidances. These information sheet guidances summarize FDA’s inspection and disqualification processes and have been updated to include procedural improvements added since 1998, when these information sheet guidances were last revised. (May and June 2010, respectively)
- Institutional Review Board (IRB) Continuing Review After Clinical Investigation Approval – Draft Guidance. (13)FDA developed this draft guidance to provide more detail on the criteria, process, and frequency of continuing review and thereby assist IRBs, sponsors, and clinical investigators in protecting the rights and welfare of study subjects. (January 2010)
Improvements to FDA’s Internal Procedures
- FDA’s Staff Manual Guide (SMG) Chapter on Debarment Actions.(14) FDA has authority under section 306 of the Federal Food, Drug, and Cosmetic Act, as a remedial measure, to debar (or prohibit) persons who have been convicted of specific felonies or misdemeanors from participating in certain FDA-regulated activities, such as providing any services to a firm that has a pending or approved new drug application. This SMG describes the procedures and timeframes for conducting a regulatory hearing to determine if an individual (e.g., a clinical investigator who has been convicted of a felony) should be debarred. (March 2009)
- Warning Letter Initiative.(15) FDA is initiating a pilot program that establishes a 15 business day timeframe for the submission of post-inspection responses to FDA 483 observations. FDA will conduct a detailed review of any timely responses received before issuing a warning letter. If, after reviewing a firm’s timely response, FDA determines a warning letter is necessary, the warning letter will acknowledge receipt of the response and reply as to the apparent adequacy of the firm’s described corrective actions. The purpose of this program is to facilitate the timely issuance of warning letters, promote prompt correction of violations, and promote efficient use of agency resources. FDA will assess the pilot after approximately 18 months to determine whether to make the program permanent. (August 2009)
- Enhanced Transparency of FDA’s Disqualification/Debarment Actions. FDA has taken steps to ensure that sponsors and IRBs involved in the development and oversight of new medical products have ready access to information about FDA's disqualification and debarment actions. FDA has developed a single webpage(16) where links to all pending and completed disqualification proceedings can be found. For debarment proceedings, FDA lists proposals to debar(17) (on the same website as proposals to disqualify) and debarred persons. (18) By improving access to information about all debarment and disqualification proceedings, FDA hopes to increase transparency and enhance protection of the public health.(19) (August 2009)
- Prioritization of Clinical Trial Inspections. FDA Centers are developing new approaches for improving the process for selecting clinical investigators and other entities for inspection, both at the pre-approval stage and earlier in the product development process. The Center for Drug Evaluation and Research (CDER) is piloting a tool that uses a sponsor-submitted clinical analysis dataset and assesses clinical sites associated with an application for inspection using a risk-based prioritization process model. This tool also has automated features to streamline the inspection request process, and is expected to provide a more efficient, analytical, and timely approach to clinical site inspection and site monitoring. Similarly, the Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER) have early intervention programs whereby ongoing studies are identified for inspection using risk-based criteria. Such studies may involve vulnerable patient populations, novel technologies, or investigational products with a potentially large public health impact (e.g., vaccines). Shifting more resources to inspections of ongoing studies allows the agency to identify potential problems while studies are in progress, thus allowing the inspected party to implement corrective actions to minimize risks to subjects and preserve the integrity of the clinical trial.
Clinical Trial Transformation Initiative (CTTI) (20)
In 2007, FDA and Duke University formed a public-private partnership in order to identify practices that, if broadly adopted, would increase the quality and efficiency of clinical trials. CTTI is composed of representatives from government, industry, patient advocacy groups, professional societies, and academia.
To achieve this goal, CTTI conducts projects to generate empirical information about how clinical research is currently conducted and to identify and test ways to improve quality and efficiency. An overview of current CTTI projects appears below.
Effective and Efficient Monitoring
The goal of this project is to identify best practices and develop sensible criteria to help sponsors choose the most appropriate monitoring methods for a trial, thereby improving quality while optimizing the deployment of resources. The project team has explored the range of monitoring practices in use and the factors driving their adoption. Various stakeholders convened last fall to reach consensus about key quality objectives for monitoring. The next step is to evaluate the various practices' strengths and weaknesses in meeting quality objectives over a range of clinical trial settings.
Improving SAE Reporting to IND Investigators
This project is generating empirical evidence about the current U.S. system for reporting serious adverse events (SAEs) to investigators under an investigational new drug (IND) application, with a goal of considering potential system modifications to more efficiently and effectively inform investigators of such events. The project includes five subprojects:
- Documenting the range of sponsor practices for reporting unexpected SAEs to investigators and for oversight of product safety (e.g., safety committees)
- Quantifying investigators’ time spent receiving, interpreting, and communicating individual expedited reports and assessing perceived value to investigators of individual expedited reports in updating a product’s risk profile
- Comparing the current practice of submitting individual SAEs to an alternative approach based on a European Commission’s guidance
- Studying patient expectations about monitoring and communicating product safety during the conduct of a clinical trial
- Convening an expert group to integrate results and recommend ways to optimize reporting of SAEs to investigators and ensure subject protection
Recent CTTI collaborations include: (21)
- An Expert Meeting on Comparative Effectiveness. In collaboration with the Pragmatic Approaches to Comparative Effectiveness (PACE) Initiative and the Center for Medical Technology Policy (CMTP), CTTI convened an expert meeting to discuss the use of randomized controlled clinical trials for comparing the effectiveness of medical products and procedures. The discussion focused on issues including improvements in operational efficiency, use of Bayesian adaptive principles in trial design and ways in which more pragmatic trial designs might be used to increase the generalizability of results. (May 2009)
- Clinical Investigator Training Course. FDA and CTTI co-sponsored a 3-day training course for clinical investigators on scientific, ethical, and regulatory aspects of clinical trials. (November 2009) The next course is tentatively planned for November 2010.
- Standards for Collecting Information about Cardiovascular Events. This collaborative pilot project is intended to develop standard definitions and data collection methods for cardiovascular events in clinical trials (e.g., a standard Case Report Form for cardiovascular endpoint events). This effort may improve uniformity of data collection and analysis of results, and thereby allow better identification of safety signals and trends during the development of new biologics, devices, and drugs.
International harmonization, capacity-building, and outreach activities
FDA conducted numerous programs to train other countries’ governments and international health regulatory bodies on GCP or to assist them in establishing or improving their GCP inspectional capacity. These activities include:
- Providing advanced GCP inspection training for member regulators from Asia Pacific Economic Cooperation (APEC; (22) Bangkok, Thailand, March 2009)
- Community Research Forum, presented collaboratively with Thailand’s Food and Drug Administration for the research community in northern Thailand (Chiang Mai, March 2009)
- Advanced and Implementation phase GCP inspection training for the government of India (Mumbai, June 2009, and Hyderabad, June 2010, respectively)
- Basic GCP “Train the Trainers” inspection training for China’s State Food and Drug Administration (Chengdu, April 2010)
- Fact-finding mission (Moscow, October 2009), signing of a Letter of Intent (May 2010), and planning for a first “Train the Trainers” inspection workshop (August 2010) for Russia’s Roszdravanadzor (Federal Service on Surveillance in Health and Social Development of the Russian Federation)
- Providing speakers and discussants to international stakeholder groups, including Drug Information Association (DIA) Euromeeting (Berlin, March 2009 and Monaco, March 2010), DIA Latin American Congress of Clinical Research (Mexico City, September 2009), DIA-China (Beijing, November 2009), and the 35th Brazilian Congress of Pharmaceutical Medicine sponsored by the Sociedade Brasileira de Medicina Farmaceutica (Sao Paulo, November 2009)
FDA-EMA Good Clinical Practices (GCP) Initiative.(23) FDA’s Center for Drug Evaluation and Research and the EMA launched a bilateral GCP Initiative, designed to ensure that clinical trials submitted in drug marketing applications in the United States and Europe are conducted uniformly, appropriately and ethically. The 18-month pilot began on September 1, 2009, and is focused on collaborative efforts to inspect clinical trial sites and studies involving pharmaceutical products.
Key objectives of the initiative include sharing information on future inspections, inspection procedures, and inspection outcomes as well as “best practices,” and keeping each other informed of GCP-related legislation, regulatory guidance and related documents. Since the pilot began, FDA and the EMA have shared inspectional information on dozens of applications and have collaborated on joint and observational inspections. In addition, the two agencies have posted information about the program on the internet(24) and have developed various operational documents and templates to enhance mutual understanding of each agency’s respective processes. At the conclusion of the pilot phase, FDA and the EMA will jointly assess the program’s scope, processes, and progress.
- Exception from Informed Consent Requirements for Emergency Research – Final Guidance. This final guidance is intended to assist sponsors, clinical investigators, and IRBs in the development, conduct, and oversight of research involving FDA-regulated products (e.g., drugs, biological products, devices) in emergency settings when an exception from the informed consent requirements is requested under 21 CFR 50.24. In particular, the guidance clarifies FDA’s expectations related to planning and conducting community consultation and public disclosure activities, and the establishment of informed consent procedures to be used when feasible.
- Guide to Informed Consent - Draft Guidance. This document will describe in detail basic and additional elements of informed consent and will include topics such as review of patient records, children as subjects, and subject participation in more than one study.
- Investigational New Drug Applications (INDs) – Determining Whether Human Research Studies Can Be Conducted Without an IND – Draft Guidance. This draft guidance is intended to assist clinical investigators, sponsors, and sponsor-investigators in determining whether human research studies must be conducted under an IND. With certain exceptions, clinical investigations in which a drug is administered to human subjects must be conducted under an IND as required in part 312. This guidance describes when an IND is required, specific situations in which an IND is not required, and a range of issues that, in FDA’s experience, have been the source of confusion or misperceptions about the application of the IND regulations.
- Financial Disclosure by Clinical Investigators -- Draft Guidance. This updated guidance will respond to recommendations(25) aimed at strengthening FDA’s oversight and review of clinical investigators’ financial disclosures. Specifically, the draft will describe: (1) the sponsor's responsibility to collect the information prior to an investigator participating in a study and ensure that all required forms and attachments are submitted in marketing applications; (2) what is meant by “due diligence” in obtaining financial disclosures from investigators; and (3) how FDA will review financial disclosure information. The guidance will also seek comment on the circumstances under which FDA should consider public release of financial disclosure information related to an approved marketing application.
- Compliance Program Guidance Manual (CPGM) chapter on Inspection of Sponsors, Contract Research Organizations (CROs), and Monitors (7348.810). This chapter is being revised to incorporate recommendations(26) for improving communications among FDA staff before, during, and after an inspection and to more clearly define the thresholds for initiating regulatory actions against non-compliant sponsors or CROs. This chapter will also include revised procedures to verify that sponsors are obtaining and maintaining required financial disclosures from investigators, and updates (if appropriate) within one year of completion of the covered trial.
- IND Safety Reporting Requirements for Human Drug and Biological Products and Safety Reporting Requirements for Bioavailability and Bioequivalence Studies in Humans – Final Rule. This revision of the safety reporting requirements for human drug and biological products subject to an IND will codify the agency’s expectations for timely review, evaluation, and submission of relevant and useful safety information and implements internationally harmonized definitions and reporting standards. The revisions will improve the utility of IND safety reports, reduce the number of reports that do not contribute in a meaningful way to the developing safety profile of the drug, expedite FDA’s review of critical safety information, better protect human subjects enrolled in clinical trials, subject bioavailability and bioequivalence studies to safety reporting requirements, promote a consistent approach to safety reporting internationally, and enable the agency to better protect and promote public health. An accompanying draft guidance has been developed and will publish with the rule.
- Acceptance of Clinical Studies Conducted Outside the U.S. - Notice of Proposed Rulemaking.(27) FDA is proposing to amend its regulations on acceptance of data from clinical studies conducted outside the United States in support of research and marketing applications for medical devices. The proposed rule would require that these studies be conducted in accordance with good clinical practice (GCP). FDA proposes to define GCP as a standard for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials in a way that provides assurance that the data and reported results are credible and accurate, and that the rights, safety, and well-being of trial subjects are protected. GCP also would include review and approval by an independent ethics committee (IEC) before initiating a study, continuing IEC review of ongoing studies, and obtaining and documenting freely given informed consent of study subjects. The proposed rule is intended to update the standards for the acceptance of clinical studies and to help to continue to ensure the protection of human subjects and the quality and integrity of data obtained from these studies.
- Disqualification of a Clinical Investigator – Notice of Proposed Rulemaking.(28) FDA is pursuing an amendment to the regulations to extend clinical investigator disqualification to include all FDA-regulated investigational products. Under this proposal, an investigator determined by FDA to be ineligible to receive a particular investigational product is also deemed to be ineligible to receive any FDA-regulated investigational product. This proposal responds to recommendations by the Government Accountability Office and harmonizes existing investigator disqualification regulations.
- Good Laboratory Practice for Non-Clinical Laboratory Studies – Advanced Notice of Proposed Rulemaking.(29) Nonclinical studies have changed markedly since issuance of the good laboratory practices (GLP) regulation (21 CFR 58) in 1978. In recognition of this change, FDA is seeking comment on whether to amend the regulation. Based on the agency’s review of the 1978 rule and preliminary stakeholder input, FDA believes that requiring nonclinical facilities/laboratories to follow a risk-based GLP quality system will help ensure the integrity of data in nonclinical studies. Although many of the requirements of the existing regulation are consistent with a GLP quality system, FDA is proposing modifications to incorporate all basic elements needed for a GLP quality system consistent with internationally recognized quality systems. FDA believes that implementation of a GLP quality system would institute a risk-based approach, reduce regulatory burden, and encourage science-based technology.
BIMO Inspections for Fiscal Year 2009
Each year, FDA's field staff conduct on-site inspections of BIMO establishments, including sponsors, monitors, clinical investigators, IRBs, and laboratories that conduct nonclinical safety studies (including animal toxicity studies) to support FDA-regulated research. The agency performs these inspections to evaluate the inspected party's practices and procedures and to determine compliance with applicable regulations. Summary information about FDA's domestic inspectional activities for fiscal year 2009 is presented below:
BIMO Inspections Completed
FY 2009
| Center | CI | IRB | Spon/Mon | GLP | Total |
|---|---|---|---|---|---|
| CBER | 83 | 15 | 11 | 6 | 115 |
| CDER * | 458 | 102 | 73 | 36 | 669 |
| CDRH | 163 | 79 | 59 | 4 | 305 |
|
CFSAN |
0 | 0 | 0 | 1 | 1 |
| CVM | 26 | na | 4 | 15 | 45 |
| All Centers | 730 | 196 | 147 | 53 | 1135 |
* + 137 BEQ Inspections (CDER specific) - total = 1272
Figure 1
Figure 1 depicts the total number of inspections that were completed by the Office of Regulatory Affairs' field investigators as well as the numbers completed for each of the assigning Centers. (BEQ denotes bioequivalence studies.)
Figure 2
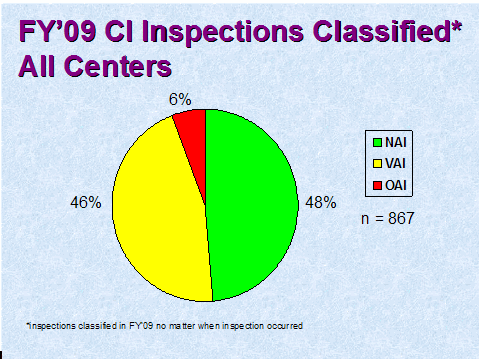
Figure 2 depicts the compliance classification of Establishment Inspection Reports (EIRs) for inspections of clinical investigators that were classified by the assigning Center in FY 2009. (NAI denotes No Action Indicated, VAI denotes Voluntary Action Indicated, and OAI denotes Official Action Indicated.)
Figure 3
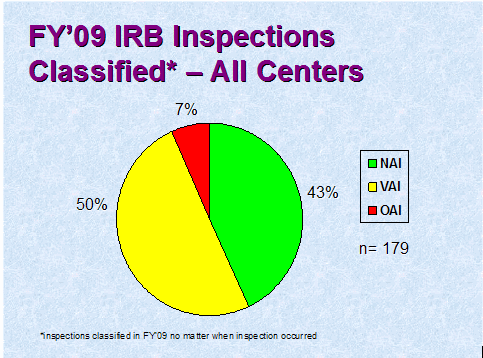
Figure 3 depicts the compliance classification of EIRs for inspections of IRBs that were classified by the assigning Center in FY 2009.
Figure 4
Figure 4 depicts the compliance classification of EIRs for inspections of sponsors that were classified by the assigning Center in FY 2009.
Figure 5
Figure 5 depicts the compliance classification of EIRs for inspections of bioequivalence studies that were classified in FY 2009.
1. The HSP/BIMO Council, which is overseeing this initiative, includes representatives from each of FDA's Centers, the Office of Regulatory Affairs (ORA), and the Office of the Commissioner (OC). These progress reports include HSP/BIMO accomplishments and initiatives from all FDA Centers and Offices.
2. The first HSP/BIMO Initiative progress report can be found at: FDA's HSP/BIMO Initiative Accomplishments - Update, March 25, 2009
3. 21 CFR 56.106.
4. Final Rules for Expanded Access to Investigational Drugs for Treatment Use and Charging for Investigational Drugs
6. Fed.Reg. 74, 68750, December 29, 2009
7. Fed. Reg.75, 7412, February 19, 0108
8. Frequently Asked Questions – IRB Registration
9. Investigator Responsibilities — Protecting the Rights, Safety, and Welfare of Study Subjects
10. Frequently Asked Questions – Statement of Investigator (Form FDA 1572)
11. Clinical Investigator Administrative Actions – Disqualification
12. FDA Inspections of Clinical Investigators
13. IRB Continuing Review After Clinical Investigation Approval
14. SMG 7712; Debarment Proceedings
15. Federal Register: August 11, 2009 (Volume 74, Number 153)
16. Good Clinical Practice: Compliance and Enforcement
17. Notice of Opportunity for Hearing (NOOH) Listing
18. FDA Debarment List (Drug Product Applications)
19. Press Release: FDA Enhances Speed and Transparency of Actions Taken Against Misconduct in Drug and Device Development
20. Clinical Trials Transformation Initiative website
21. CTTI Collaborations Web page
22. Representatives from the following APEC nations participated: Brunei, Chile, Indonesia, Malaysia, Peru, Philippines, Saudi Arabia, Singapore, South Korea, Taiwan, Thailand, and Vietnam.
23. Press Release: FDA, European Medicines Agency Launch Good Clinical Practices Initiative
25. See the Office of the Inspector General (OIG), Department of Health and Human Services report, The Food and Drug Administration’s Oversight of Clinical Investigators’ Financial Information (OEI-05-07-00730)
26. See the OIG report, The Food and Drug Administration's Oversight of Clinical Trials (OEI-01-06-00160)
27. Notice of Proposed Rulemaking: Human Subject Protection; Acceptance of Clinical Studies Conducted Outside the United States
28. Notice of Proposed Rulemaking: Disqualification of a Clinical Investigator
29. Advance Notice of Proposed Rulemaking: Good Laboratory Practice for Nonclinical Laboratory Studies