2021 FDA Science Forum
LC-HRMS Based Analytical Platform to Determine Nitrosamines in Pharmaceuticals: Modern Analytical Techniques Meet Regulatory Needs
- Authors:
- Center:
-
Contributing OfficeCenter for Drug Evaluation and Research
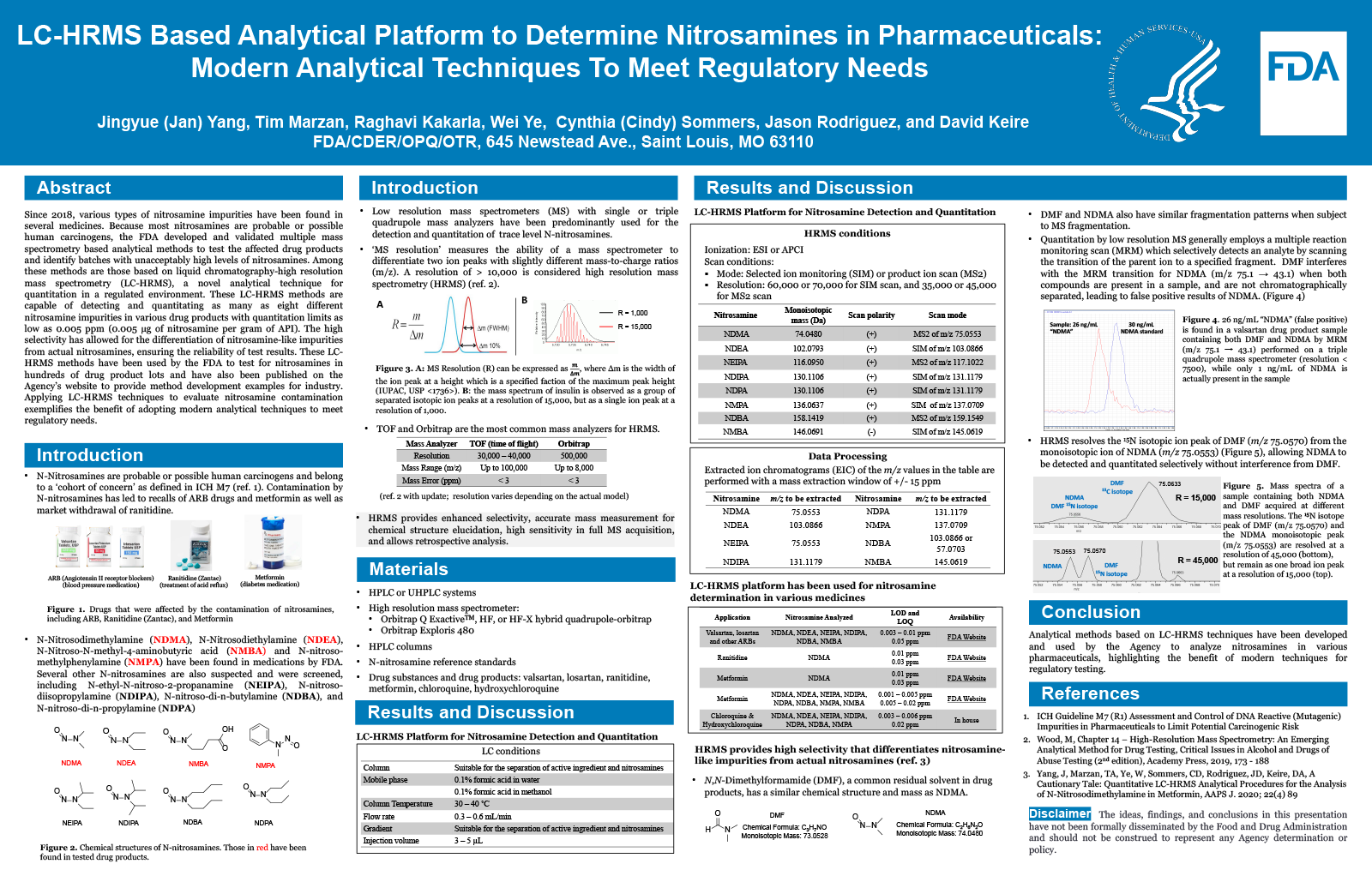
Abstract
Background
Nitrosamines are probable human carcinogens. Since 2018, certain types of nitrosamines have been found in medicines including various sartans (treatment of hypertension and heart failure), ranitidine (treatment of acid reflux) and metformin (treatment of type II diabetes). In response, the FDA collected these drug products from various manufacturers and developed methods to identify batches with unacceptable levels of nitrosamines. Trace levels of nitrosamines in these products were detected and quantitated primarily by liquid chromatography – high resolution mass spectrometry (LC-HRMS) analytical methods. LC-HRMS was chosen because of the selectivity provided by accurate mass and the appropriate sensitivity for chemical compounds at parts per million level or lower. The application of LC-HRMS in handling nitrosamine contamination issues exemplifies the benefit of adopting advanced techniques for regulatory testing.
Purpose
To develop and apply analytical methods for the determination of trace levels of nitrosamine impurities in various drug products.
Methodology
The methods were developed based on liquid chromatography-high resolution mass spectrometry (LC-HRMS) techniques. Nitrosamines were separated from active pharmaceutical ingredients (API) by LC with subsequent detection and quantitation by the high mass resolution and accurate mass capabilities of HRMS.
Results
Multiple LC-HRMS methods were developed and validated to detect and quantitate as many as seven different nitrosamine impurities in various drug products. The developed methods are capable of quantitating as little as 0.005 µg of nitrosamine per gram of API, meeting the requirement of low limit quantitation. Ideally, an LC-HRMS method for one drug product can be adapted for the screening of nitrosamines in a separate drug product. The inherent selectivity of LC-HRMS methods allows for the differentiation of nitrosamine-like impurities from actual nitrosamines, ensuring the reliability of the reported values. These methods have been used by the FDA to test for nitrosamines in hundreds of drug product lots. They have also been published on the Agency’s website to provide method development examples for industry.
Conclusion
Analytical methods based on LC-HRMS techniques have been developed and used by the Agency to analyze nitrosamines in various pharmaceuticals, highlighting the benefit of modern techniques for regulatory testing.