Drug Trials Snapshots: SCENESSE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the SCENESSE Package Insert for complete information.
SCENESSE (afamelanotide)
sē-nĕs’
Clinuvel Inc.
Approval date: October 8, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
SCENESSE is a drug for increasing pain-free light exposure in adult patients with a history of reactions to light (phototoxicity) from erythropoietic protoporphyria (EPP).
EPP is a rare, inherited disease that generally starts early in life. Among other characteristics, patients with this disease have painful phototoxic episodes which lead to light avoidance and chronic skin changes.
How is this drug used?
SCENESSE is an implant administered under the skin (subcutaneously) every 2 months, by a healthcare professional.
What are the benefits of this drug?
Over 180 days, patients who received the SCENESSE implant spent more time (about 64 hours) in direct sunlight with no pain in comparison to patients who received placebo (about 41 hours).
What are the benefits of this drug (results of trials used to assess efficacy)?
Efficacy results from two trials are presented below.
| In Trial 1, the primary endpoint was the total number of hours over 180 days spent in direct sunlight between 10 am and 6 pm on days with no pain. The median total number of hours over 180 days spent in direct sunlight between 10 am and 6 pm on days with no pain was 64.1 hours for subjects receiving SCENESSE and 40.5 hours for subjects receiving vehicle. |
| In Trial 2, the primary endpoint was the total number of hours over 270 days spent outdoors between 10 am and 3 pm on days with no pain for which “most of the day” spent in direct sunlight. The median total number of hours over 270 days spent outdoors between 10 am and 3 pm on days with no pain for which “most of the day” was spent in direct sunlight was 6.0 hours for subjects in the SCENESSE group and 0.75 hours for subjects in the vehicle group. |
SCENESSE Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: SCENESSE worked similarly in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in how well SCENESSE worked among races could not be determined.
- Age: The majority of patents were adults 18 to 65 years old. The number of patients older than 65 years was limited; therefore, differences in how well the drug worked in patients younger and older than 65 years could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup efficacy analyses for each trial are presented below.
Table 1. Total Hours of Direct Sun on Days with No Pain by Subgroup in Trial 1 (ITT-Diary Card Population)
|
|
SCENESSE |
Vehicle |
|
|
Sex |
|||
|
Men |
N |
28 |
20 |
|
Women |
N |
18 |
23 |
|
Race |
|||
|
White |
N |
45 |
41 |
|
Age |
|
||
|
≥18 to < 65 years |
N |
45 |
40 |
|
≥65 years |
N |
1 |
3 |
Table 2. Total Hours of Direct Sun on Days with No Pain by Subgroup in Trial 2 (ITT Population)
|
|
SCENESSE |
Vehicle |
|
|
Sex |
|||
|
Men |
N |
17 |
20 |
|
Women |
N |
21 |
16 |
|
Race |
|||
|
Caucasian |
N |
38 |
35 |
|
Age |
|||
|
≥18 to < 65 years |
N |
36 |
36 |
|
≥65 years |
N |
2 |
|
ITT=Intent-to-treat
Adapted from FDA Review
What are the possible side effects?
The most common side effects of SCENESSE are implant site reaction, nausea, throat pain, cough, fatigue, dizziness, skin darkening, sleepiness, mole (melanocytic nevus), respiratory tract infection, sleepiness, non-acute porphyria, and skin irritation.
Because of skin darkening (including darkening of moles and freckles), a regular skin examination is recommended.
What are the possible side effects (results of trials used to assess safety)?
Adverse reactions occurring in more than 2% of patients from pooled trials are presented below.
Table 3. Adverse Reactions Occurring in >2% of Patients with EPP Through Month 6 (safety population)
|
Adverse Reaction |
SCENESSE |
Vehicle |
|
Implant site reaction1 |
26 (21%) |
12 (10%) |
|
Nausea |
24 (19%) |
17 (14%) |
|
Oropharyngeal pain |
9 (7%) |
6 (5%) |
|
Cough |
8 (6%) |
4 (3%) |
|
Fatigue |
7 (6%) |
3 (3%) |
|
Skin hyperpigmentation2 |
5 (4%) |
0 (0%) |
|
Dizziness |
5 (4%) |
4 (3%) |
|
Melanocytic nevus |
5 (4%) |
2 (2%) |
|
Respiratory tract infection |
5 (4%) |
3 (3%) |
|
Somnolence |
3 (2%) |
1 (1%) |
|
Non-acute porphyria |
2 (2%) |
0 (0%) |
|
Skin irritation |
2 (2%) |
0 (0%) |
1Implant site reaction includes: implant site bruising, discoloration, erythema, hemorrhage, hypertrophy, irritation, nodule, pain, pruritus, swelling; injection site bruising and erythema; and expelled implant
2Skin hyperpigmentation includes skin hyperpigmentation, pigmentation lip (subject also had skin hyperpigmentation), and pigmentation disorder.
SCENESSE Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among other races could not be determined
- Age: The majority of patients were adults 18 to 65 years old. The number of patents older than 65 years was limited; therefore, differences in the occurrence of side effects between patients younger and older than 65 years could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Differences in overall adverse events by sex are summarized below. Differences among race and age groups were not tested because of predominantly White population 18-65 years of age.
Table 4. Treatment Emergent Adverse Events (TEAE) Severity by Sex in the Safety Population
|
|
SCENESSE
|
Vehicle |
||
|
Women |
Men |
Women |
Men |
|
|
TEAE Severity, n (%) |
||||
|
Mild |
49 (86%) |
48 (71%) |
46 (79%) |
38 (62%) |
|
Moderate |
29 (51%) |
24 (35%) |
28 (48%) |
25 (41%) |
|
Severe |
12 (21%) |
5 (7%) |
8 (14%) |
8 (13%) |
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved SCENESSE based on evidence from three clinical trials (Trial 1/ NCT 01605136, Trial 2/ NCT00979745 and Trial 3/ NCT01097044) of 244 adult patients 18-74 years of age with EPP. The trials were conducted at 22 sites in US and Europe.
Figures below summarize demographics of all three trials that provided data for assessment of side effects (safety population).
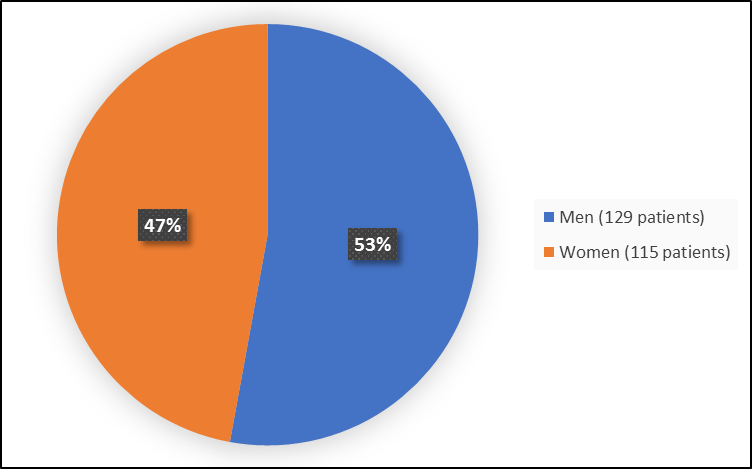
Figure 1 summarizes how many men and women were in the clinical trials used to evaluate side effects.
Figure 1. Baseline Demographics by Sex
FDA Review
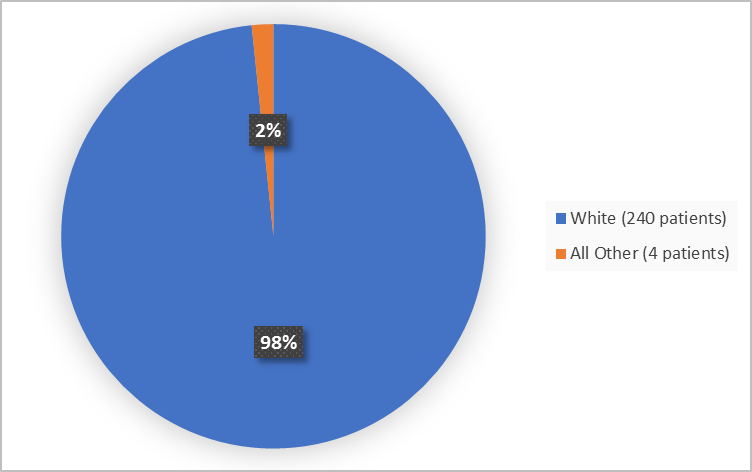
Figure 2 summarizes the percentage of patients by race in the clinical trials used to evaluate side effects.
Figure 2. Baseline Demographics by Race
FDA Review
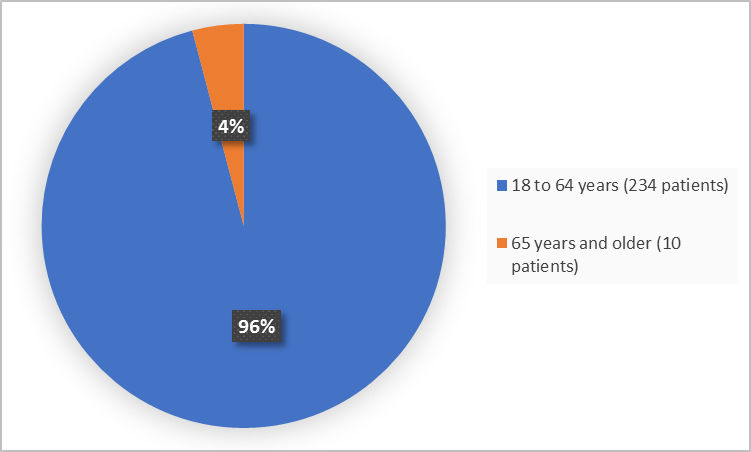
Figure 3 summarizes the percentage of patients by age in the clinical trials used to evaluate side effects.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
Presented below is pooled population from all three trials (safety population).
Table 5. Demographics in Safety Population (Pooled Trials 1, 2 and 3)
|
Demographic Characteristic |
SCENESSE |
Vehicle |
Total |
|
Sex |
|
|
|
|
Men |
68 (54%) |
61 (51%) |
129 (53) |
|
Women |
57 (46%) |
58 (49%) |
115 (47) |
|
Race |
|||
|
White |
124 (99%) |
116 (98%) |
240 (98) |
|
Asian |
0 |
1 (1%) |
1 (0.4%) |
|
American Indian or Alaska Native or Pacific Islander |
0 |
1 (1%) |
1 (0.4%) |
|
Other |
1(1%) |
1 (1%) |
2 (1%) |
|
Age |
|||
|
Min, max (years) |
18, 70 |
18, 74 |
18, 74 |
|
Age Group |
|
|
|
|
≥ 18 - < 65 years |
121 (97%) |
113 (95%) |
234 (96%) |
|
≥ 65 years |
4 (3%) |
6 (5%) |
10 (4%) |
|
Ethnicity-not collected |
|||
|
Country |
|
|
|
|
United States |
87 (70%) |
83 (70%) |
170 (70%) |
|
Outside United States |
38 (30%) |
36 (30%) |
74 (30%) |
Adapted from FDA Review
How were the trials designed?
There were three trials that evaluated SCENESSE in patients with EPP.
In Trial 1, patients received SCENESSE or vehicle implant every 2 months and were followed for 180 days. Patients recorded every day the number of hours spent in direct sunlight and whether they experienced any phototoxic pain that day. The trial measured the total number of hours over 180 days spent in direct sunlight between 10 am and 6 pm on days with no pain.
In Trial 2, patients received SCENESSE or vehicle implants every 2 months and were followed for 270 days. Patients recorded every day the number of hours spent outdoors whether “most of the day” was spent in direct sunlight, shade, or a combination of both, and whether they experienced any phototoxic pain that day. The trial measured the total number of hours over 270 days spent outdoors between 10 am and 3 pm on days with no pain for which “most of the day” was spent in direct sunlight.
In Trial 3 patients were randomized to receive a total of three SCENESSE or vehicle implants administered subcutaneously every 2 months and were followed for 180 days. Data from this trial were used primarily for assessment of side effects.
How were the trials designed?
In Trial 1, patients were randomized to receive a total of three SCENESSE or vehicle implants administered subcutaneously every 2 months and were followed for 180 days. Patients recorded every day the number of hours spent in direct sunlight between 10 am and 6 pm, the number of hours spent in shade between 10 am and 6 pm, and whether they experienced any phototoxic pain that day. The primary endpoint was the total number of hours over 180 days spent in direct sunlight between 10 am and 6 pm on days with no pain.
In Trial 2, patients were randomized to receive a total of five SCENESSE or vehicle implants administered subcutaneously every 2 months and were followed for 270 days. Patients recorded every day the number of hours spent outdoors between 10 am and 3 pm, whether “most of the day” was spent in direct sunlight, shade, or a combination of both, and whether they experienced any phototoxic pain that day. The primary endpoint was the total number of hours over 270 days spent outdoors between 10 am and 3 pm on days with no pain for which “most of the day” was spent in direct sunlight.
In Trial 3 patients were randomized to receive a total of three SCENESSE or vehicle implants administered subcutaneously every 2 months and were followed for 180 days. Data from this trial were used primarily for assessment of side effects.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.