| 1 |
|
Enhancing Systematic Literature Review with Advanced AI: A Study on LLM-Based Screening |
Dan Li, Leihong Wu, Svitlana Shpyleva, Ting Li, Joshua Xu |
dan.li@fda.hhs.gov |
| 2 |
|
Protecting Public Health Using (Quantitative) Structure-Activity Relationships and Expert Knowledge: Considerations for ICH M7 Class 4 Impurities |
Barbara Scott, Naomi Kruhlak, Jahan Cooper, Naomi SwederGold, Samuel Odebamowo |
barbara.scott@fda.hhs.gov |
| 3 |
|
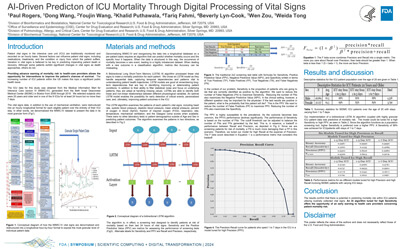
AI-Driven Prediction of ICU Mortality Through Digital Processing of Vital Signs |
Paul Rogers, Dong Wang, Youjin Wang, Khalid Puthawala, Tariq Fahmi, Beverly Lyn-Cook, Wen Zou, Weida Tong |
paul.rogers@fda.hhs.gov |
| 4 |
|
PrecisionFDA: A Sandbox for Innovative and Collaborative Artificial Intelligence (AI) Solutions for Public Health |
Amy Gutierrez, Adrienne Phifer, Samuel Westreich, Ezekiel Maier, Gabrielle Kosoy, Melissa Moss, Tanav Thanjavuru, Omar Serang, Michael Morgan, Samir Lababidi, Elaine Johanson |
adrienne.phifer@fda.hhs.gov |
| 5 |
|
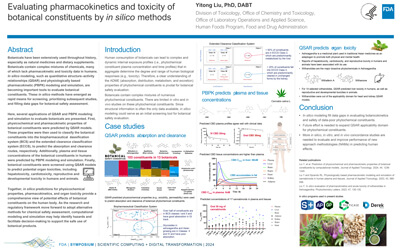
Evaluating pharmacokinetics and toxicity of botanical constituents by in silico methods |
Yitong Liu |
yitong.liu@fda.hhs.gov |
| 6 |
|
Enhancing Public Health and Regulatory Efficiency with Microsoft’s Power Platform |
LaToya Crabbe, Gavin Obrien |
latoya.crabbe@fda.hhs.gov |
| 7 |
|
Low-resolution image reconstruction methodology using Generative Adversarial Network (GAN) to improve drug product quality assessment |
Jayanti Das, Theophilus Acquah, Yuan Zhang, Yang, Yang, Muhammad Ashraf, Geng Tian, Xiaoming Xu |
jayanti.das@fda.hhs.gov |
| 8 |
|
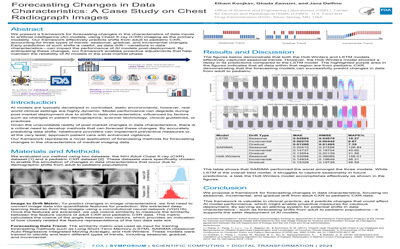
Forecasting Changes in Data Characteristics: A Case Study on Chest Radiograph Images |
Elham Konjkav, Ghada Zamzmi, Jana Delfino |
elham.konjkav@fda.hhs.gov |
| 9 |
|
AskFDALabel: Enhancing AE Detection, Profiling, Classification, and Monitoring |
Leihong Wu, Joshua Xu, Hong Fang, Weida Tong |
leihong.wu@fda.hhs.gov |
| 10 |
|
Design and development of digital phantoms to provide guidance for simulations of electrophysiological activity in tissue |
Aseem Milind Pradhan, Richard Gray, Abouzar Kaboudian |
abouzar.kaboudian@fda.hhs.gov |
| 11 |
|
Optimizing Applications for Expedited Risk Assessment on Amazon Web Services (AWS) at the FDA |
Hong Yang, Yin Huang, Rebecca Kahn, Mark Walderhaug, Jason Claeys, Richard Forshee |
rebecca.kahn@fda.hhs.gov |
| 12 |
|
Application of AI/ML for risk assessments on Antisense Oligonucleotides (ASOs) aggregation |
Xiangyi Dong, Muhammad Ashraf, Haiou Qu, Deyi Zhang, Olen Stephens, Lawrence Perez, Maxwell Korang-Yeboah |
xiangyi.dong@fda.hhs.gov |
| 13 |
|
Use of Artificial Intelligence to Improve the Calculation of Percent Adhesion for Transdermal and Topical Delivery Systems |
Chao Wang, Caroline Strasinger, Yu-Ting Weng, Xutong Zhao |
chao.wang@fda.hhs.gov |
| 14 |
|
Dataforward - Data Science Upskilling Program For FDA Staff |
Tomas Drgon |
tomas.drgon@fda.hhs.gov |
| 15 |
|
A Comparative Analysis of Statistical Methods for Detecting Emerging Chemical Signals Utilizing NIH Grant Data |
Amirreza Nickkar, Ernest Kwegyir-Afful |
amirreza.nickkar@fda.hhs.gov |
| 16 |
|
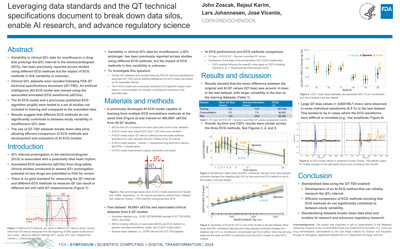
Leveraging data standards and the QT technical specifications document to break down data silos, enable AI research, and advance regulatory science |
John Zoscak, Rejaul Karim, Lars Johannesen, Jose Vicente Ruiz |
jose.vicenteruiz@fda.hhs.gov |
| 17 |
|
FDA Innovative Technologies and Advanced Manufacturing Hub |
Kirstie Snodderly, Jordan Veney, Jamie Elliott, C. Kent Jordan, Benjamin Jones, General Lee, James C. Coburn |
james.coburn@fda.hhs.gov |
| 18 |
|
Performance Evaluation of Deep Learning-based Tumor-Infiltrating Lymphocyte Cell Detection Algorithms for Histopathology Whole Slide Images |
Arian Arab, Lakshyana KC, Seyed Kahaki, Weijie Chen |
weijie.chen@fda.hhs.gov |
| 19 |
|
Summarizing FAERS Narratives with Generative AI: Methods, Resource Requirements, and Quality Assessment |
Kate Dowdy, Anna Hoffman, Tyree Giles, David Kugele, Jacqueline Guill1, Isaac Chang, Gregory Jackson |
katherine.dowdy@fda.hhs.gov |
| 20 |
|
Advancing CAPA analysis with NLP: unlocking organizational insights and drawing on past improvements |
Evgeny Kiselev, Danielle Larese, Selen Stromgren |
evgeny.kiselev@fda.hhs.gov |
| 21 |
|
A Machine Learning Approach for Balancing Risk and Time For Evaluation of Import Entry Filers |
Revati Rajane, Eckart Bindewald, Lija Fellows, Indu Konduri, Tyler Poppenwimer, John Jackson, Serajus Salaheen, Robert Timmons, Isaac Garcia, Brandon Tao, Ben Duan, Faiad Rahaman, Precise Software Solutions, FDA Office of Regulatory Affairs |
eckart.bindewald@fda.hhs.gov |
| 22 |
|
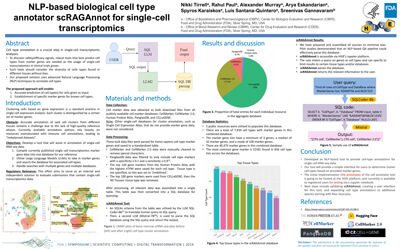
ENLP-based biological cell type annotator scRAGAnnot for single-cell transcriptomics |
Nikki Tirrella, Rahul Paula, Alexander Murraya, Arya Eskandariana, Spyros Karaiskosa, Luis Santana-Quinteroa, Sreenivas Gannavaram |
nikki.tirrell@fda.hhs.gov |
| 23 |
|
AI Assisted Tool for Regulatory Document Conformance Review - EIR Pilot Performance |
Anna Hoffman, Daniel Priver, Ian Baker, Brian Campbell, Leslie Jackanicz, Jaqueline Guill, Indu Konduri |
anna.hoffman@fda.hhs.gov |
| 24 |
|
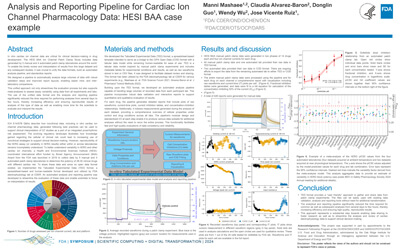
Analysis and Reporting Pipeline for Cardiac Ion Channel Pharmacology Data: HESI BAA case example |
Manni Mashaee, Claudia Alvarez-Baron, Donglin Guo, Wendy Wu, Jose Vicente Ruiz |
manni.mashaee@fda.hhs.gov |
| 25 |
|
Reproducibility in AI – a case study |
Ting Li, Kamel Mansouri, Weida Tong |
ting.li@fda.hhs.gov |
| 26 |
|
Proposed Assessment of Trends in Disarities in PAD Outcomes |
Osman Yogurtcu, Olamide Alabi, Misti Malone, Artur Belov, Orestis Panagiotou, Danica Marinac-Dabic, Gregory Pappas |
osman.yogurtcu@fda.hhs.gov |
| 27 |
|
Leveraging Machine Learning to Characterize Relationships Among Biosimilar Product Quality Attributes and Clinical Performance |
Kevin McCoy, Stella Grosser, Jinhui Zhang, Mack Shih, Xiaoming Xu, Junghi Kim |
junghi.kim@fda.hhs.gov |
| 28 |
|
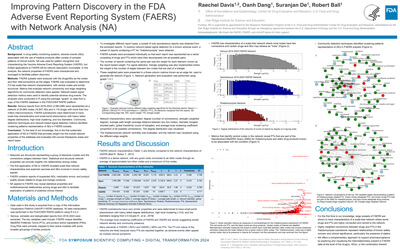
Improving Pattern Discovery in the FDA Adverse Event Reporting System (FAERS) with Network Analysis (NA) |
Raechel Davis, Oanh Dang, Suranjan De, Robert Ball |
raechel.davis@fda.hhs.gov |
| 29 |
|
Pathogen detection in human blood and plasma using long read sequencing |
Sean Smith, Moussa Kourout, Scott Espich, Carolyn Fisher, Irina Tiper, Anjan Purkayastha, Luis Santana-Quintero, Robert Duncan |
sean.smith@fda.hhs.gov |
| 30 |
|
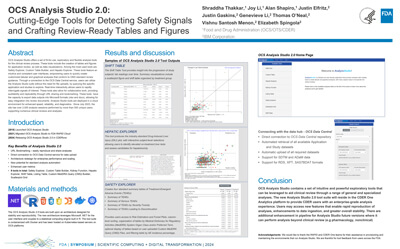
OCS Analysis StudioV2: Cutting-Edge Tools for Detecting Safety Signals and Crafting Review-Ready Tables and Figures |
Shraddha Thakkar, Joy Li, Alan Shapiro, Justin Elfritz, Justin Gaskins, Genevieve Li, Thomas O’Neal, Vishnu Santosh Menon, Elizabeth Spingola |
shraddha.thakkar@fda.hhs.gov |
| 31 |
|
FDA Insights: Simple interactive access to FDA information for public, industry, clinical research Using customized LLMs and RAG |
Chetan “Aryan” Paul, Ravichandran Sarangan, Ramprasad Venkatraman |
chetan.paul@fda.hhs.gov |
| 32 |
|
Scalable Workflow for Genome Binning on HPC Clusters |
Saul Sarria, Mike Mikailov, Fu-Jyh Luo, Daniel Tadesse, Kenny Cha, Caitlin Welsh |
saul.sarria@fda.hhs.gov |
| 33 |
|
Scalable Assessment of Progression-Free Survival in Metastatic Breast Cancer using HPC Clusters |
Mike Mikailov, Subrata Mukherjee, Qian Cao, Fu-Jyh Luo, Kenny Cha |
mike.mikailov@fda.hhs.gov |
| 34 |
|
Medical Device Demand Modeling for Supply Chain Analysis |
Rachel Townsley, Siddhartha Nambiar, Michael Vella, Alexandra Cha, Tammy Beckham |
rachel.townsley@fda.hhs.gov |