The Over-the-Counter Drug Facts Label
FDA requires a standard label of important drug information for all over-the-counter (OTC) drug products
Always Read the Label
Reading the product label is the most important part of taking care of yourself or your family when using nonprescription medicines, also known as OTC medicines. These medicines are available without a prescription. It is especially important to read the label of OTC medicines because they can be taken without seeing a doctor. FDA regulation makes sure the labels on all OTC medicines (from a bottle of sunscreen to a bottle of cough syrup) have information listed in the same order; are arranged in a consistent style and contain easier to understand words.
What's On The Label
All nonprescription, OTC medicine labels have detailed usage and warning information so consumers can properly choose and use the products.
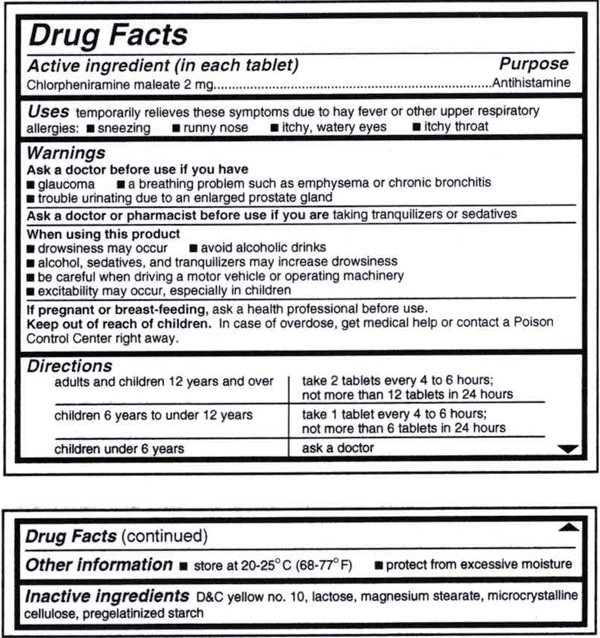
Below is an example of what the OTC medicine label looks like.
Disclaimer: This label is for illustrative purposes only.
What It Means
Click to learn more:
Active Ingredient(s). The substance(s) in the product that cause the intended therapeutic effect. This also states the amount of substance(s) for each dosage unit.
Purpose(s). Product action or pharmacologic category.
Use(s). Symptoms or diseases the product will treat or prevent.
Warning(s). When not to use the product; conditions that may require advice from a doctor before taking the product; possible interactions or side effects; when to stop taking the product and when to contact a doctor; pregnancy or breastfeeding warnings; when it is appropriate to talk to a doctor or pharmacist; keep product out of children’s reach.
Directions. Dosage instructions-- how much to take, how to take, how often, and how long to take the drug.
Inactive Ingredients. Substances such as binders, colors or flavorings.
Other Information. How to store the product properly and information about certain ingredients (such as the amount of calcium, potassium, or sodium the product contains).
Note: The Drug Facts labeling requirements do not apply to dietary supplements, which are regulated as food products, and are labeled with a Supplement Facts panel.
The Label Also Tells You...
- The expiration date, when applicable (date after which you should not use the product). If there is no expiration date on the label, the drug should be considered expired three years after purchase.
- Lot or batch code (manufacturer information to help identify the product).
- Name and address of manufacturer, packer, or distributor.
- Net quantity of contents (how much of the product is in each package).
Reading the Label: The Key to Proper Medicine Use
The label tells you what a medicine is supposed to do, who should or should not take it, and how to use it. But efforts to provide good labeling can't help unless you read and use the information. It's up to you to be informed and use your medicines wisely and responsibly.
The manufacturers of OTC medicines sometimes make changes to their products or labeling (new ingredients, dosages, or warnings). In addition, many OTC medicines may have similar brand names with different active ingredients. Make sure to read the label each time you use the product. Always look for special "flags" or "banners" on the front product label alerting you to such changes. If you read the label and still have questions, ask your doctor, pharmacist, or other health care professional for advice.
Tamper-Evident Packaging: An Important Safety Feature
The makers of OTC medicines widely use tamper-evident packaging for their products. This is to help protect consumers against possible criminal tampering. Drug products with tamper-evident packaging have a statement on the packaging describing this safety feature. It is always important to inspect the outer packaging before you buy an OTC drug product and to look at the product again before you take it.
OTC medicines are sold in containers with child safety closures. Use them properly. Remember—keep all medicines out of the sight and reach of children.