Medical Countermeasures (MCMs)
The FDA coordinates medical countermeasure (MCM) development, preparedness, and response.
MCM Activities

MCM Legal, Regulatory & Policy Framework
Helping ensure that U.S. laws, regulations and policies support preparedness and response for potential CBRN and emerging infectious disease threats.
Preparedness Research
Supports FDA’s public health emergency preparedness and response mission—including MCMi— by advancing discovery and innovation in regulatory science research.

MCM Issues
MCM-related emergency preparedness and response topics, including COVID-19, mpox, Ebola, antimicrobial resistance, smallpox, pediatric MCMs, and more.

Outreach and Professional Development
Training courses and activities to help FDA meet the regulatory challenges posed by new science and technology developments.
MCM News and Info

About MCMs
Learn more about how the FDA coordinates MCM development, preparedness, and response.

MCM Newsletter
Subscribe to receive the latest FDA preparedness and response updates.

MCM Initiative Program Update
Learn more about how FDA works every day to protect national health and security, and support public health emergency preparedness.

COVID-19 Updates from FDA
The latest COVID-19 news from FDA, plus frequently asked questions and resources for consumers, health care providers, medical product sponsors, and other stakeholders.
Popular Links

Emergency Use Authorization (EUA)

Expiration Dating Extension
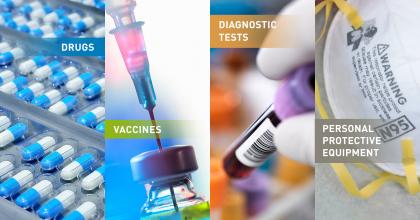
What are Medical Countermeasures?

