Domestic Mutual Reliance (Food)
Domestic Mutual Reliance is a seamless partnership that enables the FDA and states with comparable regulatory public health systems, as trusted partners, to rely on, coordinate with, and leverage one another’s work, data, and actions to meet the public health goal of a safe national food supply.
The FDA works with our state partners to build and recognize high quality programs using nationally recognized regulatory program standards like Manufactured Food Regulatory Program Standards (MFRPS) and the Animal Feed Regulatory Program Standards (AFRPS). Such collaboration provides opportunities for the FDA and state partners to lay a quality foundation for sharing information and working together on regulatory services and food protection that industry and consumers can trust.
Domestic Mutual Reliance Work by Division
- Reinforces federal and state information sharing systems to protect industry trade secrets and other non-public information
- Improves quality, integrity and reliability of inspections, investigations, laboratory results, and technical support to promote voluntary compliance and rapid response when necessary
- Promotes workforce development and equivalency of regulatory authorities based on most current science nationwide
- Focuses on prevention efforts by coordinating allocation of inspection and sampling resources on high-risk products and non-compliant operations before illnesses occur and impact the whole industry
- Reduces duplication of inspections, sampling, and compliance activities to minimize impact on a firm’s resources
- Encourages proactive sharing and leveraging of industry expertise through food safety and inspection outreach and education
- Coordinates food safety and inspection resources and support for new businesses to promote a food safety culture and encourage active managerial control
- Supports joint development of IT platforms for sharing information not only among regulatory partners, but also with industry
- Improves response preparedness and coordination, surveillance, investigation, mitigation, and post-response activities to support short- and long-term foodborne illness prevention efforts
- Improves quality and sharing of root cause analysis to support development of effective prevention initiatives with all food safety stakeholders
- Establishes a collaborative infrastructure in an integrated food safety system to detect and address new risks and emerging food safety issues
- Increases detection capabilities and methodologies that can be shared with private and industry laboratories
- Reduces risk and liability associated with human and animal foodborne illness outbreaks, contamination, and recalls
- Improves industry and consumer confidence in regulatory programs
- Improves consumer confidence in the regulatory/industry system of checks and balances
- Improves consumer confidence in nation’s food supply, which is good for everyone!
Success includes collaboration on ten operational areas:
- Information Sharing and Public Health Protection through Legal Authority
- Planning and Evaluation
- Leveraging Resources
- Information Technology
- Training
- Risk-Based Inspection Program
- Compliance and Enforcement
- Industry and Community Relations
- Laboratory Support
- Emergency Response
Download an Infographic on the Ten Operational Areas (PDF)
- August 2024 - The FDA signed a partnership agreement with the Michigan Department of Health & Rural Development to strengthen public health by fostering interaction and cooperation for the ongoing development of an Integrated Food Safety System (IFSS). Partners will formalize domestic mutual reliance for the regulatory oversight of human and animal food establishing streamlined and efficient processes to enhance collaboration on information sharing, emergency response, evaluation activities, and other prevention, detection, and response activities that directly impact food safety and public health.
- August 2024 - Kudos to NY Partnership Agreement partners on advancing domestic mutual reliance. OHAFO_E1 recently shared that the New York State Agriculture & Markets (NYSAGM) has begun using FDA full PC inspections to help them meet their inspection frequency and better reallocate their field resources. The partners are beginning to explore non-contract inspection (NCI) data acceptance by FDA under their partnership agreement goals.
- April 2024 – Year 3 Accomplishments of the Laboratory Flexible Funding Model (LFFM) are available. The LFFM supports an integrated national laboratory network that operationalizes domestic mutual reliance in support of an integrated food safety system.
- April 2024 – The Missouri Department of Health and Senior Services (MO DHSS) recently embargoed imported coffee creamer due to undeclared allergens. MO DHSS and the FDA’s Human and Animal Food West Division 2 team worked with the firm to recondition the product with a new label to bring the product into compliance. After several failed attempts by the firm to correct the label, MO DHSS witnessed the destruction of the product at a landfill to prevent the product from being distributed back into the marketplace.
- February 2024 – The FDA worked with the Alaska Department of Environmental Conservation Laboratory to update an infographic highlighting the accomplishments from FY2017-FY2023 of their Specialized Partnership Agreement, in which Alaska analyzes radionuclides on the FDA’s behalf.
- February 2024 –As part of an environmental investigation of elevated blood lead levels in children, the North Carolina Department of Health and Human Services and North Carolina Department of Agriculture and Consumer Services tested apple cinnamon fruit puree pouches and found extremely high concentrations of lead, which can cause acute toxicity. The FDA was notified, and all three agencies shared documentation of their findings. The FDA then proactively issued a consumer advisory, and the company recalled the product. The FDA also activated the Food Emergency Response Network to mobilize state Laboratory Flexible Funding Model-funded labs for additional testing in support of the investigation. To date, 18 state laboratories have tested more than 160 products in response to this incident and shared results with the FDA. As of February 2, 2024, the CDC reported more than 300 cases linked to this product. However, due to the quick action among state and federal partners, the product was identified and removed from commerce – thus preventing additional exposures and adverse effects.
January 2024 – The FDA produced a video depicting domestic mutual reliance “in action” among the FDA, states, and other regulatory partners. It focuses on foodborne illness prevention, detection, response activities, and public health impacts.
- December 2023 – The Association of Food and Drug Officials and Quality Assurance Magazine recently published a three-part webinar series on the integrated food safety system featuring, among others, the FDA’s Office of Partnerships Director Barbara Cassens (part 2), Assistant Commissioner for Partnerships and Policy Erik Mettler (part 3), and multiple key FDA partners. The series addressed vision, obstacles, and how ORA supports partners to operationalize domestic mutual reliance, including the use of partnership agreements (part 3).
- October 2023 – The FDA signed a partnership agreement with the Guam Department of Public Health and Social Services and the Guam Customs and Quarantine Agency. This agreement formalizes the agencies’ shared commitment to public health and consumer protection. It includes activities related to the oversight of imported foods, imported drugs including opioids, medical devices, biological products, tobacco products, and cosmetics to help prevent fraudulent and potentially dangerous products from entering supply chains.
- October 2023 – The FDA announced the signing of a partnership agreement with the Pennsylvania Department of Agriculture’s Bureau of Food Safety & Laboratory Services that will focus on inventory reconciliation, reducing foodborne illness risk, and minimizing duplication of work. The state of Pennsylvania played an integral role in reaching the recent milestone for accepting 1,000 non-contract inspections.
- July 2023 - The FDA worked closely with state regulatory partners in Florida, Iowa, Pennsylvania, Utah, Virginia, and Wisconsin to achieve the major milestone of accepting 1,000 non-contract inspections. Also known as NCIs, these non-high-risk inspections are conducted by the state under its own authority in which no other regulatory action was required. Similarly, these states have been able to leverage a similar number of FDA inspections to support their oversight activities. NCIs greatly increase efficiency and minimize duplication of efforts for both regulators and industry. These efficiencies enable the FDA and our state regulatory partners to maximize the impact of millions of dollars in state and federal funding, thereby avoiding additional burden to the American taxpayer and enabling regulators to expand coverage and focus limited resources on areas of highest risk to advance food safety for consumers. Learn more about NCIs in this Partnership for Food Protection story.
June 2023 - Overview of the Recall Integration Partnership Project
This brief video describes the Recall Integration Partnership Project (RIPP), which enhances communication and understanding of FDA/state roles and responsibilities during recall events to more effectively leverage regulatory and enforcement actions that protect public health. Visit the RIPP website today to learn more: https://www.pfp-ifss.org/ripp/.
- May 2023 – The FDA announced the signing of a developmental partnership agreement with the Puerto Rico Department of Consumer Affairs (DACO). The agreement will focus on emergency response, partner training, and minimizing duplicative efforts.
- March 2023 – The latest Partnership for Food Protection newsletter featured two employees from the FDA’s Office of Regulatory Affairs: Division Director Tim Mueller and Consumer Safety Officer Priscilla Neves. Mueller discussed the ORA Data Exchange and its connection to domestic mutual reliance and an integrated food safety system. Neves discussed how her current role overseeing the domestic mutual reliance project in ORA enables her to collaborate with partners to advance food safety.
- February 2023 – The FDA signed a domestic mutual reliance partnership agreement with the New York Department of Agriculture and Markets. New York takes an innovative approach to collaborate on food safety for domestically produced foods, imported foods, and laboratory testing in conjunction with the FDA. This ORA News and Stories article details the agreement.
- February 2023 – In this Partnership for Food Protection story, ORA Human and Animal Food State Liaison Julie Vosilus and Chief of the Food and Consumer Safety Bureau Mark Speltz in the Iowa Department of Inspections and Appeals discuss domestic mutual reliance in Iowa, which recently signed a partnership agreement with the FDA.
- December 2022 – The FDA announced the signing of three new domestic mutual reliance partnership agreements with the Minnesota Department of Agriculture, Virginia Department of Agriculture and Consumer Services, and Iowa Department of Inspections and Appeals. The agreements will focus on inventory reconciliation, training, and work planning, among other areas. Associate Commissioner for Regulatory Affairs Judy McMeekin discusses the agreements in Food Safety Magazine.
- October 2022 – The Coordinated Outbreak Response & Evaluation (CORE) Network coordinates FDA responses during outbreak investigations. How does CORE use the incident command system with local, state, and federal partners? Learn more about domestic mutual reliance in action through the Food Safety Magazine article, "The Incident Command System and Foodborne Illness Outbreak Investigations."
- October 2022 – The FDA released a TechTalk podcast entitled “Data Exchange in the New Era of Smarter Food Safety” to discuss the platform created by the FDA’s Office of Regulatory Affairs to securely share information between the agency and regulatory partners at the state and local levels. This platform supports domestic mutual reliance and other key initiatives to ensure a safe food supply across the country.
- September 2022 – The FDA learned that an imported honey product promoted and sold for sexual enhancement had been seized by the Fresno County Sheriff’s Office for presumptive methamphetamine. Though confirmatory results were negative for methamphetamine, a search revealed that the FDA had previously issued an import alert for the product for containing sildenafil, the active ingredient in Viagra®. Because the shipping paperwork suggested the product was being sold to a grocery store in New York, the FDA alerted the NY State Department of Agriculture and Markets (NYSAGM). NYSAGM responded and 144 sachets of this product were seized and destroyed under signed waiver during the investigation.
- July 2022 – The West Virginia Department of Agriculture analyzed tahini samples from grocery stores, which were collected by the state’s health department as part of a joint Rapid Response Team preparedness exercise. Laboratory analysis found the samples to contain Salmonella. Notification was provided to the West Virginia Department of Health and Human Resources, which referred this to its counterpart in New Jersey, where the product’s importer and distributor was located. In coordination with West Virginia and the FDA, the New Jersey Department of Health (NJDOH) reached out to the firm. At the urging of NJDOH, the firm chose to conduct a voluntary recall. As a result, the NJDOH did not pursue any additional enforcement actions, i.e., embargo of their products and inspection/investigation of multiple locations.
- August 2022 – The FDA Winchester Engineering Analytical Center (WEAC) participated in an exercise sponsored by Rhode Island Emergency Management Agency (RIEMA), per a partnership agreement between the two, to evaluate the readiness and capability to respond to a nuclear incident. RIEMA collected and delivered samples to WEAC, and WEAC performed gamma-ray analysis and reported data to REIMA. All participants achieved the exercise objectives, and WEAC delivered analytical results within two hours of getting the samples.
- August 2022 – The FDA created an infographic explaining our partnership with the Alaska Department of Environmental Conservation.
- July 2022 – The FDA produced a video providing an overview of domestic mutual reliance and how we work with states to advance food safety.
- The FDA, CDC, and state and local partners worked together to investigate a multistate outbreak of Listeria infections linked to Dole packaged leafy greens that sickened 18 people in 13 states. The FDA analyzed positive samples collected by Dole from harvesting and processing equipment. Results showed that the Listeria strain from the equipment matched both the strain causing the illnesses and the strain from product samples collected by the Michigan Department of Agriculture and Rural Development during the December 2021 outbreak investigation, and a routine sample collected by the Georgia Department of Agriculture prior to the investigation. Dole issued two voluntary recalls, stopped using the equipment, and temporarily halted production at two processing facilities. As of April 4, 2022, the CDC has declared the outbreak over.
- June 2022 – The Maryland State Department of Health Laboratories Administration analyzed samples of freeze-dried blueberries collected from grocery stores. Lab analysis found that one product contained a high amount of lead (for which there is no established action or regulatory level in this food). The FDA determined that the blueberries represented a hazard to consumers and referred the sample to the California Department of Public Health (CDPH), where the product’s distributor was located. CDPH followed up with the firm, which voluntarily recalled the product and implemented additional quality assurance processes regarding heavy metals.
- April 2022 – A joint inspection by the FDA and the New York State Department of Agriculture and Markets resulted in the seizure and destruction of nearly 5,000 pounds of rodent-defiled food. The filth conditions had been discovered during a routine FDA inspection of a firm in Brooklyn. The FDA coordinated with the state to leverage individual authorities. Thanks to this collaboration, food deemed unfit for human consumption was prevented from distribution to consumers.
- March 2022 – The state of Connecticut and the FDA worked closely together regarding an asparagus sample imported from Peru that was found to contain carbofuran, a potentially harmful pesticide that is not allowed at any level in asparagus. The Connecticut Division of Consumer Protection and the Connecticut Agricultural Experiment Station collected and tested the asparagus. The FDA used the state’s test results and traceback information to add the foreign processor to the FDA’s Import Alert 99-05, which subjects the firm to detention without physical examination. This sample was collected as part of the Laboratory Flexible Funding Model (LFFM) Cooperative Agreement Program.
- March 2022 – The FDA announced the signing of a domestic mutual reliance partnership agreement with the Minnesota Department of Agriculture – the first such agreement to include both human and animal food. The work will focus on data and information sharing, official establishment inventory reconciliation and maintenance, and establishing key metrics.
- February 2022 – Strong federal-state collaboration facilitated the voluntary recall of product from Royal Ice Cream. The company initiated the recall after FDA sampling revealed the presence of Listeria monocytogenes on processing equipment, and it expanded the recall after the Massachusetts Department of Public Health detected the bacteria in retail samples. Royal Ice Cream is working with the FDA to investigate the cause.
- February 2022 – In this Food Safety Magazine “Food Safety Matters” podcast, FDA Assistant Commissioner for Partnerships and Policy Erik Mettler and Assistant Commissioner for Human and Animal Food Operations Michael Rogers joined Travis Waller of the Utah Department of Agriculture and Food to discuss a “day in the life” of a domestic mutual reliance partnership agreement with the FDA.
- November 2021 – The FDA and the Florida Department of Agriculture and Consumer Services (FDACS) recently reached a major milestone of exchanging and accepting more than 500 of each other’s manufactured food inspection reports. The FDA’s enhanced IT support has greatly increased the efficiency of data sharing between the agency and state partners. These efforts capture the intent of the domestic mutual reliance partnership agreement between FDACS and the FDA, signed on October 29, 2021, to share program information that will improve public health by minimizing duplication of work and leveraging resources. For more examples of the benefits of domestic mutual reliance, visit DMR Benefits for Industry and Consumers.
- On October 7, 2021, the FDA announced the signing of four domestic mutual reliance partnership agreements with the California Department of Public Health, Florida Department of Agriculture and Consumer Services, Utah Department of Agriculture and Food, and Wisconsin Department of Agriculture, Trade and Consumer Protection Division of Food and Recreational Safety.
- October 2021 – Food Safety Magazine discusses the FDA’s new domestic mutual reliance partnership agreements with California, Florida, Utah, and Wisconsin. FDA Assistant Commissioner for Partnerships and Policy Erik Mettler is the author.
- July 2021 - Our new video highlights how the FDA’s Rapid Response Team program embodies domestic mutual reliance in action during human and animal food emergencies. The video features a case study involving the federal-state teams from Maryland and Wisconsin, posters from the annual meeting, and much more.
- May 2021 - Thanks to effective federal-state communication channels, Giant Eagle, Inc., Hy-Vee, Inc., and the Kroger Co. were able to work with the FDA to quickly issue a voluntary recall of Chicken Street Taco Kits supplied by Reser’s Fine Foods, due to the possibility the product may contain an undeclared egg allergen.
- April 2021 - Food Safety Magazine describes how domestic mutual reliance builds upon the successes of FDA’s Rapid Response Teams model in collaboration with states to improve food safety in the United States. Erik Mettler, FDA Assistant Commissioner for Partnerships and Policy, and Natalie Adan, Food Safety Division Director of the Georgia Department of Agriculture, are co-authors.
Partnership Agreements
Partnership agreements formally document our domestic mutual reliance relationships to improve foodborne illness prevention, detection, and response in an Integrated Food Safety System (IFSS). Partners collaborate in the following operational areas to build a highly trained workforce, coordinate food safety inspection efforts, share data, leverage organizational resources, focus on prevention, and better respond to outbreaks.
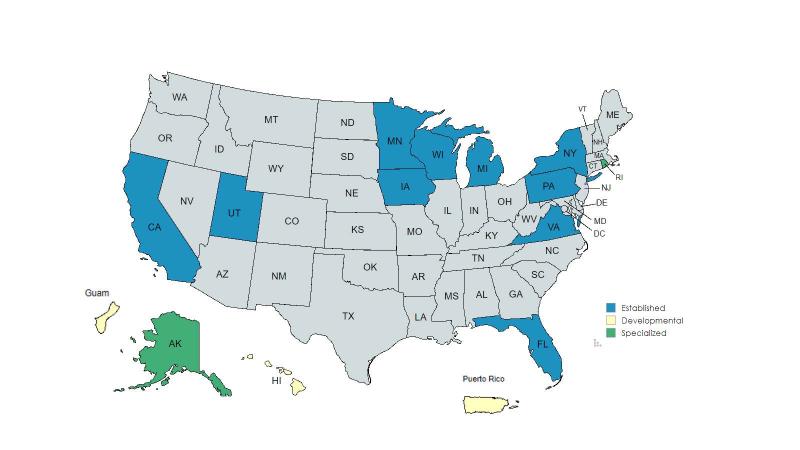
Established partnership agreements: California, Florida, Iowa, Michigan, Minnesota, New York, Pennsylvania, Utah, Virginia, Wisconsin
Developmental partnership agreements: Guam, Hawaii, Puerto Rico
Specialized partnership agreements: Alaska, Rhode Island
Resources
- Video: A Snapshot of the FDA’s Domestic Mutual Reliance Activities with Regulatory Partners
- Video: Domestic Mutual Reliance Overview
- Food Safety Modernization Act
- 20.88 Agreements Database
- Commissioning Information