Manufactured Food Regulatory Program Standards (MFRPS)
The Manufactured Food Regulatory Program Standards (MFRPS) are a critical component in establishing the national Integrated Food Safety System (IFSS). The goal of the MFRPS is to implement a nationally integrated, risk-based, food safety system focused on protecting public health. The MFRPS establish a uniform basis for measuring and improving the performance of prevention, intervention, and response activities of manufactured food regulatory programs in the United States. The development and implementation of the standards will help federal and state programs better direct their regulatory activities toward reducing foodborne illness.
The MFRPS areas of focus include:
- Standard 1 - Regulatory Foundation
- Standard 2 - Training Program
- Standard 3 - Inspection Program
- Standard 4 - Inspection Audit Program
- Standard 5 - Food-related Illness, Outbreaks and HazardsResponse
- Standard 6 - Compliance and Enforcement Program
- Standard 7 - Industry and Community Relations
- Standard 8 - Program Resources
- Standard 9 - Program Assessment
- Standard 10 - Laboratory Support
View the 2022 MFRPS Summary of Changes.
Frequently Asked Questions
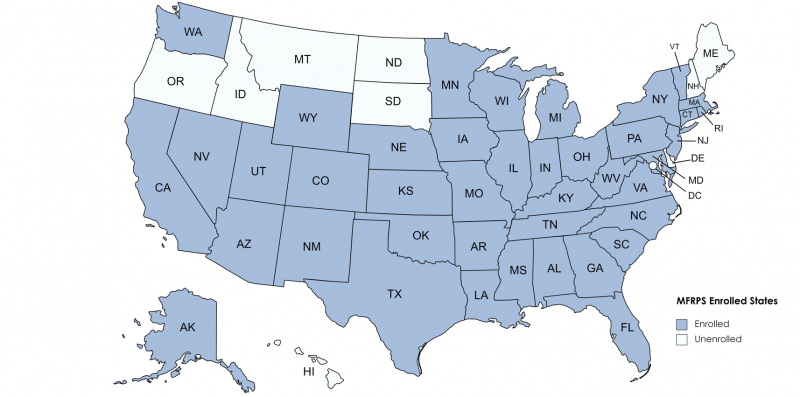
Which states have a regulatory agency(s) currently enrolled in the MFRPS?
United States Map displaying 41 states currently enrolled in the MFRPS: AK, AL, AR, AZ, CA*, CO, CT, FL, GA, IA, IL, IN, KS, KY, LA, MA, MD, MI, MN, MO, MS, NC, NE, NJ, NM, NV, NY, OH OK, PA, RI, SC*, TN, TX, UT, VA, VT, WI, WV*, WA, WY
- There are 44 state programs currently enrolled.
- CA, SC and WV each have two agencies with MFRPS cooperative agreements (Health and Agriculture).
- Over $9.7M in funding under this cooperative agreement program.
Is funding available to assist states in implementing the MFRPS?
More information about this funding program can be obtained by viewing the latest Funding Opportunity Announcement (FOA).
States' manufactured food regulatory programs receiving funds under this cooperative agreement are expected to achieve significant to full conformance by Year 5 of the cooperative agreement. Eligible entities include states and their manufactured food regulatory programs with current FDA food safety inspection contracts or those that agree to enter into a food contract with FDA at the earliest possible date.
NOTE: The FDA reduced the funding levels for the MFRPS track, the Dietary Supplement track and the Special Projects track was very limited this year. The FDA has a tighter budget for the 2023-24 year. Therefore, we have moved all grantees to the old MFRPS Funding Levels per RFA-FD-18-001: Flexible Funding Model - Infrastructure Development and Maintenance for State Manufactured Food Regulatory Programs (U18) (nih.gov):
Level 1: $150,000
Level 2: $225,000
Level 3: $300,000
$15,000 FPTF track
$50,000 Dietary Supplement track
What other resources are available to help state programs implement MFRPS?
The OP DSI is responsible for conducting education and outreach to state programs on MFRPS. ORA provides funding to the Association of Food and Drug Officials to administer the Manufactured Food Regulatory Program Alliance (MFRPA) which is responsible for providing additional training and collaboration to support standards development and implementation.
States' manufactured food regulatory programs receiving funds under this cooperative agreement are expected to achieve significant to full conformance by Year 5 of the cooperative agreement. Eligible entities include states and their manufactured food regulatory programs with current FDA food safety inspection contracts or those that agree to enter into a food contract with FDA at the earliest possible date.
All state personnel actively involved in MFRPS should register for a FoodSHIELD account and join the MFRPS Workgroup in FoodSHIELD.
Register for a FoodSHIELD Account