Investigation of Avian Influenza A (H5N1) Virus in Dairy Cattle
The U.S. Department of Agriculture (USDA), the U.S. Food and Drug Administration (FDA), and the Centers for Disease Control and Prevention (CDC), along with state partners, continue to investigate an outbreak of Avian Influenza A (H5N1) impacting poultry, dairy cows, and people in multiple states.
The responsibility of the FDA is to protect the public health by ensuring the safety of the milk, dairy products, and animal feed supply. The U.S. Department of Agriculture’s Animal and Plant Health Inspection Service is leading the response from the animal health perspective while coordinating closely with the FDA and with the Centers for Disease Control and Prevention.
Status of the Milk Supply What's New
FDA H5N1 Communications | Research & Studies
Resources for Producers | Resources for Consumers
After the first detection of HPAI H5N1 in dairy cattle in March 2024, the FDA has engaged in research and other efforts with industry, federal, and state partners to ensure the continued effectiveness of the federal-state milk safety system.
Nearly all (99%) of the commercial milk supply that is produced on dairy farms in the US comes from farms that participate in the Grade A Milk Safety Program and follow the Pasteurized Milk Ordinance (PMO), which includes controls that help ensure the safety of dairy products. Pasteurization and diversion or destruction of abnormal milk are two important measures that are part of the federal-state milk safety system.
The process of pasteurization has helped to ensure the health of the American public for more than 100 years. Pasteurization kills harmful bacteria and viruses by heating milk to a specific temperature over time. Even if the virus is detected in raw milk, the current pasteurization process (HTST – High Temperature, Short Time) will inactivate the virus.
October 3, 2024
States are invited to participate in a new study that will generate data to aid in the understanding of the prevalence of H5N1 in bulk raw cow’s milk received by dairy processing facilities across the nation. Dubbed “the silo study,” the information garnered from this research can help inform the national strategy to control the spread of the virus to other dairy cattle and avian flocks, and to identify any viral transformations. See Research & Studies for additional information.
Sign-up to receive Constituent Updates to stay informed.
The FDA and USDA co-signed letters and conducted outreach to the dairy processing industry and dairy retailers to communicate about the safety of the commercial milk supply during the Highly Pathogenic Avian Influenza A (H5N1) outbreak impacting dairy cows in multiple states. The letters review the studies that confirm pasteurization inactivates H5N1 and protects public health. The letters emphasize the confidence the FDA and USDA have that pasteurization is effective in inactivating H5N1 in raw milk just as it is for pathogens against which we began pasteurizing raw milk 100 years ago.
Dairy Processing Industry Letter (PDF: 287KB)
Retail Industry Letter (PDF: 287KB)
The FDA’s Center for Veterinary Medicine announced the establishment of four Animal and Veterinary Innovation Centers (AVICs), which are the recipients of funding for work to advance regulatory science and further development of innovative products and approaches to better support animal health and veterinary interventions. One of these AVICs is the University of Wisconsin-Madison, for research to explore the use of genome editing in chickens to reduce susceptibility or provide resistance to HPAI and other avian viruses by genetically targeting pro-viral host factors, antiviral proteins, or viral genes. These AVICs further the goals outlined in the FDA’s Animal and Veterinary Innovation Agenda (AVIA), which communicated the agency’s plans to spur innovation to better protect human and animal health.
Today the FDA issued a letter to state, territorial, local and tribal health partners regarding the sale and consumption of raw milk as part of the agency’s ongoing work to protect both human and animal health during the outbreak of Highly Pathogenic H5N1 Avian Influenza (H5N1 HPAI) virus in dairy cattle.
While the introduction into interstate commerce of raw milk for human consumption is prohibited under the FDA’s authority, we know that a number of states permit the intrastate (within the state’s borders) sale of raw milk for human consumption, with varying structures and requirements to these state programs.
The FDA wants to collaborate with our regulatory partners in reducing exposure of viable HPAI H5N1 to humans and animals during this outbreak in dairy cattle. The agency’s letter to all state, territorial, and tribal partners provides recommendations to help reduce risks of exposure to HPAI H5N1 virus associated with raw milk consumption.
The FDA’s longstanding recommendations regarding the consumption of unpasteurized (raw) milk is that it is considered a high-risk food because it has the potential to be contaminated with pathogens that cause illness and it has been linked to numerous foodborne illness outbreaks in the past. Based on the limited research and information available, we do not know at this time if the HPAI H5N1 virus can be transmitted to humans through consumption of raw milk and products made from raw milk from infected cows. Pasteurization is a proven process with a long history of protecting public health and is highly effective at eliminating the dangers associated with consuming raw milk.
The FDA, alongside our federal and state partners, is continuing to take a stepwise approach to our scientific analysis of commercial milk safety during the first-of-its-kind detection of HPAI H5N1 in dairy cattle. While our initial assessment of the milk safety system continues to be affirmed by sampling and testing of retail dairy products, there remain a number of collective activities being undertaken to ensure the continued effectiveness of the federal-state milk safety system. The FDA will continue to follow a sound scientific process to inform the agency’s public health decisions related to food safety.
Last week we announced preliminary results of a study of 297 retail dairy samples, which were all found to be negative for viable virus. The FDA is today announcing that all final egg inoculation tests associated with this retail sampling study have been completed and were also found to be negative for viable HPAI H5N1 virus. These confirmatory test results mark the completion of our laboratory research efforts related to these 297 retail dairy samples. Additional sampling and other surveillance activities will continue.
While our retail sampling test results to date are clear about the safety of the commercial milk supply and representative of real-world scenarios, additional scientific work is being undertaken to validate the criteria for pasteurization relative to the HPAI H5N1 virus and will include tests using pasteurization equipment typically used by milk processors. Today, we’d like to share more about our additional research efforts.
The established pasteurization process set forth in federal regulation (21 CFR 1240.61) and the Pasteurized Milk Ordinance (PMO) provides specific temperature and time requirements for effective elimination of known pathogens in the milk supply. To further validate pasteurization effectiveness against this recently detected virus, the FDA previously noted it was testing samples of pooled raw milk routed for commercial processing to characterize potential virus levels that the pasteurization process must eliminate. Our pasteurization study is designed to better replicate real-world conditions to deliver the pasteurization treatment parameters set forth in the CFR and PMO, and to assess their effectiveness in inactivating HPAI H5N1 in bovine milk and other dairy products.
The results from this study will help further the FDA’s understanding of pasteurization efficacy against anticipated concentrations of virus under real-world processing conditions. The pasteurization study is ongoing and we anticipate making preliminary results available in the near future.
Today, the agency is also announcing an additional $8 million is being made available to support its ongoing response activities to ensure the safety of the commercial milk supply. This funding will support the agency’s ability to validate pasteurization criteria, conduct surveillance at different points in the milk production system, bolster laboratory capacity and provide needed resources to train staff on biosecurity procedures.
Additionally, these funds will help support HPAI H5N1 activities in partnership with state co-regulatory partners, who administer state programs as part of the federal/state milk safety system. It may also allow the FDA to partner with universities on critical research questions.
To date, the totality of evidence – including studies on the effectiveness of pasteurization against multiple pathogens, recent studies on the effectiveness of pasteurization of HPAI H5N1 in eggs at lower temperatures than generally used in dairy products, negative retail sample results to date, and real-world evidence from the last 100 years of the PMO — continues to indicate that the commercial milk supply is safe.
At the same time, the FDA also continues to advise against the consumption of raw milk (milk that has not been pasteurized). The FDA and CDC have long standing information regarding the increased risk of foodborne illness associated with numerous pathogens that may be present in raw milk. This increased risk exists for both humans and other animals that might drink raw milk. Additional guidance on raw milk and milk handling can be found on our website.
We are committed to continuing to initiate, support, and collaborate on research and surveillance of milk production, processing, and pasteurization to further our public health goals.
Since the onset of the Highly Pathogenic Avian Influenza A H5N1 (H5N1) outbreak in dairy cattle earlier this year, researchers across the federal and state governments have been working to understand the prevalence of H5N1 in the U.S. milk supply. This week, states were invited to participate in a new study that will generate data to aid in the understanding of the prevalence of H5N1 in bulk raw cow’s milk received by dairy processing facilities across the nation. Dubbed “the silo study,” the information garnered from this research can help inform the national strategy to control the spread of the virus to other dairy cattle and avian flocks, and to identify any viral transformations.
Beginning October 28, Grade “A” raw cow’s milk from participating states intended to be pasteurized will be sampled from raw milk storage silos at dairy processing facilities, over a six-week period.
This double-blinded study is designed for data-gathering purposes only, with no intent or means of traceback or trace forward. Neither participating nor non-participating states or facilities will be identified as part of this project. Samples will be collected, double blinded, and sent to the U.S. Department of Agriculture (USDA) National Veterinary Services Laboratory for analysis.
A robust milk sampling program exists within the regulatory framework of the Pasteurized Milk Ordinance (PMO) and the federal-state cooperative Grade “A” milk program. The silo study seeks to leverage the Grade “A” Milk Safety Cooperative Program and its members to further understand the prevalence of HPAI in cow’s milk that is sent for commercial processing and stored at dairy processing facilities prior to pasteurization.
State participation in the silo study is voluntary, but it is hoped that states will want to contribute to this important project. The FDA, National Conference of Interstate Milk Shipments (NCIMS), and USDA will review the results of the silo study and are committed to providing study results in the near future.
The FDA’s ongoing assessments of the milk safety system continue to confirm that pasteurization is effective at eliminating infectious H5N1 virus in dairy milk. In addition to this silo study, the FDA is funding research activities (see the September 26 update) designed to ensure the continued effectiveness of the federal-state milk safety system. The FDA will continue to follow a sound, scientific process to inform the agency’s public health decisions related to milk safety during the first-of-its-kind outbreak of H5N1 in dairy cattle. The FDA remains committed to providing further updates on research efforts.
The FDA is providing an update about ongoing research efforts it is undertaking to help ensure the safety of dairy products during the outbreak of Highly Pathogenic Avian Influenza A (H5N1) virus in dairy cattle and to work towards reducing the threat of H5N1 to human and animal health.
Since outlining the FDA’s research objectives in summer 2024 through the FDA’s research agenda, the FDA has continued collaborating with our federal and academic partners to conduct the imperative work to understand the effectiveness of inactivation methods and further assure the safety of retail dairy products. Through FDA H5N1 funding and in cooperation with Cornell University, the University of Wisconsin-Madison, National Institutes of Health Rocky Mountain Laboratories (NIH-RML) and Centers of Excellence for Influenza Research and Response (NIH-CEIRR) partners, the University of Georgia and St. Jude Children’s Research Hospital, the FDA is advancing the following applied research:
Thermal Inactivation Kinetic Studies: In collaboration with Cornell University, NIH-RML, and the University of Georgia, the FDA will determine the effects of various time and temperature combinations outside of standard pasteurization parameters and temperature combinations on H5N1 viability in fluid milk that will allow objective risk assessments of various dairy product manufacturing processes.
Viral Inactivation Studies: In collaboration with Cornell University, the FDA will assess H5N1 inactivation under different aged raw milk cheese manufacturing processes.
H5N1 Viability Testing: The FDA has partnered with Cornell University, the University of Georgia, and St. Jude Children’s Research Hospital to provide H5N1 virus viability testing as needed.
One Health Interventions: In collaboration with Cornell University, the FDA will investigate practical disposal measures for raw milk waste. In collaboration with the University of Wisconsin-Madison, the FDA will explore the development of genome-edited chickens to reduce susceptibility or provide resistance to H5N1 and other avian viruses.
The FDA, alongside its partners, expects to share further updates on these research efforts in the coming months. We continue to affirm the safety of the commercial milk supply by ongoing sampling and testing of retail dairy products. The agency remains committed to taking a stepwise approach to its scientific analysis of the safety of the commercial milk supply and interventions that reduce human and animal health risks.
On Aug. 13, 2024, the FDA announced the results from the agency’s second survey of retail dairy products. Samples were processed and analyzed by a USDA-Agricultural Research Services (ARS) laboratory from June 18 – July 31, 2024. All 167 samples were found to be negative for viable H5N1 virus. The FDA’s second sampling survey tested dairy products collected at retail locations, including aged raw milk cheese, pasteurized fluid milk and products made from pasteurized milk, such as pasteurized cheeses, cream cheese, butter, and ice cream.
The samples, collected by a third party, were aseptically repackaged to remove identifying manufacturing marks and then sent to USDA-ARS for extraction and analysis. USDA reported the results to the FDA. No viable virus was detected in the samples. These results strengthen previous assessments that commercial pasteurization inactivates the H5N1 virus. The study is published in the Journal of Food Protection: Inactivation of Highly Pathogenic Avian Influenza Virus with High-temperature Short Time Continuous Flow Pasteurization and Virus Detection in Bulk Milk Tanks.
The tables below provide additional details on the samples and test results for our second survey of retail dairy products.
Table 1: Breakdown of Retail Sample Results by State Where Fluid Milk Was Processed
| State Where Milk Was Processed (May Not Relate to Where Milk Was Produced) | Detection of Live Virus in Retail Product(s) | Number of Retail Product Samples Tested | Retail Product Samples Negative for Viral RNA (qRT-PCR Screening -) |
Retail Product Samples Positive for Viral RNA (qRT-PCR Screening +) |
Retail Product Sample Results for Live Virus (Viability Testing by Egg Inoculation) |
|---|---|---|---|---|---|
| AL | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| AZ | No | 1 | 1 | 0 | Not Performed (Negative qRT-PCR) |
| CA | No | 6 | 6 | 0 | Not Performed (Negative qRT-PCR) |
|
CO |
No | 3 | 2 | 1 | 0 |
| CT | No | 1 | 1 | 0 | Not Performed (Negative qRT-PCR) |
| DE | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| ID | No | 28 | 15 | 13 | 0 |
| IL | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| KY | No | 3 | 2 | 1 | 0 |
| LA | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| MD | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| ME | No | 1 | 1 | 0 | Not Performed (Negative qRT-PCR) |
|
MI |
No | 13 | 12 | 1 | 0 |
| MN |
No |
6 | 6 | 0 | Not Performed (Negative qRT-PCR) |
| MS | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| MT | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| NC | No | 5 | 5 | 0 | Not Performed (Negative qRT-PCR) |
| ND | No | 1 | 1 | 0 | Not Performed (Negative qRT-PCR) |
| NE | No | 1 | 1 | 0 | Not Performed (Negative qRT-PCR) |
| NH | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| NJ | No | 1 | 1 | 0 | Not Performed (Negative qRT-PCR) |
| NM | No | 5 | 5 | 0 | Not Performed (Negative qRT-PCR) |
| OH | No | 14 | 13 | 1 |
0 |
| PA | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| RI | No | 4 | 4 | 0 | Not Performed (Negative qRT-PCR) |
| SD | No | 6 | 5 | 1 |
0 |
| TN | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
|
TX |
No | 24 | 13 | 11 | 0 |
| VA | No | 1 | 1 | 0 | Not Performed (Negative qRT-PCR) |
| VT | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| WI | No | 10 | 10 | 0 | Not Performed (Negative qRT-PCR) |
Table 2: Breakdown of Retail Sample Results by Product Type
| Product Category |
Number of Retail Product Samples | Detection of Live Virus in Retail Product | Retail Product Samples Negative for Viral RNA (qRT-PCR Screening -) |
Retail Product Samples Positive for Viral RNA (qRT-PCR Screening +) |
Percent of Retail Product Samples Positive for Viral RNA (via qRT-PCR screening) |
Retail Product Sample Results for Live Virus (Confirmatory Virus Culture) |
|---|---|---|---|---|---|---|
| Skim Milk | 7 | No | 7 | 0 | 0% | N/A |
| 1% Milk | 11 | No | 9 | 2 | 18.2% | 0/11 |
| 2% Milk | 23 | No | 22 | 1 | 4.3% | 0/23 |
| Whole Milk | 38 | No | 33 | 5 | 13.2 | 0/38 |
| Heavy Cream | 1 | No | 1 | 0 | 0% | N/A |
| Cheddar Cheese | 14 | No | 12 | 2 | 14.3% | 0/14 |
| Mozzarella Cheese | 12 | No | 6 | 6 | 50.0% | 0/12 |
| Processed Cheese | 6 | No | 5 | 1 | 16.7% | 0/6 |
| Cream Cheese | 3 | No | 3 | 0 | 0% | N/A |
| Butter | 7 | No | 4 | 3 | 42.9% | 0/7 |
| Ice Cream | 22 | No | 13 | 9 | 40.9% | 0/22 |
| Aged Raw Cheese | 23 | No | 23 | 0 | 0% | N/A |
| Total | 167 | None | 138 | 29 | 17.4% | 0/167 |
The FDA, along with our federal partners at the U.S. Department of Agriculture, is announcing results from a first-of-its-kind study using the process typically used by commercial milk processors. The intention of this study was to further confirm that pasteurization is effective at inactivating Highly Pathogenic H5N1 Avian Influenza (H5N1 HPAI) virus in fluid milk and other dairy products made from pasteurized milk.
The study – the only one to date designed to simulate commercial milk processing – found that the most commonly used pasteurization time and temperature requirements were effective at inactivating the H5N1 HPAI virus in milk. These results complement the FDA’s initial retail sampling study (See May 10, 2024 update) in which all 297 samples of dairy products collected at retail locations were found to be negative for viable H5N1 HPAI virus.
Collectively, these studies provide strong assurances that the commercial milk supply is safe.
“This commercial milk processing simulation study is notably different than some recent benchtop studies published by other institutions which have shown mixed results regarding the sensitivity of the virus to thermal treatment,” said Stephen Walker, Ph.D., P.E., Consumer Safety Officer, in the FDA’s Center for Food Safety and Applied Nutrition. “Other recent studies relied on benchtop equipment that was likely not sufficient to accurately simulate High Temperature Short Time (HTST) processing conditions. In contrast, the results of the study announced today strongly indicate that the virus is much more sensitive to heat treatment with commercial pasteurization equipment than other studies might suggest.”
For this study, researchers first needed to understand the level of virus in unpasteurized (raw) milk that is intended for commercial processing. To do this, researchers tested 275 raw milk samples obtained from multiple farms in four affected states. The sampling was intentionally focused on regions with infected herds and the results are not nationally representative. Individual farms were eligible to be sampled multiple times during the sampling period. One-hundred and fifty-eight of the raw milk samples were positive for viral fragments and, of those, 39 were found to have infectious virus with an average concentration of 3.5 log10 EID (egg infectious doses)50 per milliliter – which is about 3,000 virus particles per milliliter.
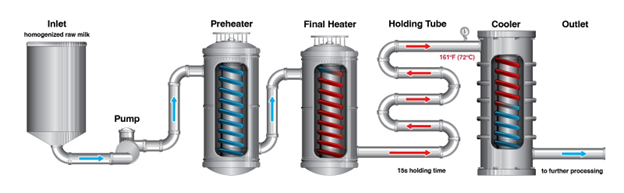
Next, researchers had to determine if the continuous-flow processing of the milk at 161°F (72°C) for 15 seconds was effective at eliminating the levels of virus found in raw milk. These treatment conditions, often referred to as “high-temperature-short-time” (HTST) or “flash pasteurization,” represent the pasteurization treatment (time/temperature combination) required by the Code of Federal Regulations (CFR) and the Pasteurized Milk Ordinance (PMO) that is most commonly utilized by the dairy industry.
In the study, scientists used homogenized raw whole milk that was artificially contaminated with a higher concentration of virus than was found in any raw milk samples – an average concentration of 6.7 log10 EID (egg infectious doses)50 per milliliter (or approximately 5 million virus particles per milliliter). High numbers of organisms are typically used when conducting inactivation studies to document high levels of inactivation. The levels are also comparable to those used in benchtop experiments.
When these spiked milk samples were processed in a HTST continuous flow pasteurization system (illustrated below), which was designed to closely simulate commercial processing conditions, in each of the total of nine repeated experiments, the virus was completely inactivated.
Milk samples that were collected mid-process indicate that the virus was inactivated very quickly, and the FDA’s process engineering experts have extrapolated that HTST pasteurization conditions are likely eliminating at least 12 log10 EID50 per milliliter (about 1 trillion virus particles per milliliter). These results establish that HTST pasteurization is effective at eliminating the virus from milk with a large margin of safety.
“While testing finished product post-pasteurization is one strategy to detect potential problems in finished products, validating the effectiveness of the pasteurization parameters critically demonstrates that commercial milk processing is capable of controlling the HPAI virus and further provides broad assurance that pasteurized milk and dairy products made from pasteurized milk are safe,” said Nathan Anderson, Ph.D., Director, Division of Food Processing Science and Technology in the FDA’s Center for Food Safety and Applied Nutrition.
Scientists at both the FDA and USDA submitted a manuscript with the study details to the Journal of Food Protection for peer review prior to publication in the journal.
The results from this and previous studies are clear about the safety of the commercial milk supply. The FDA is continuing additional surveillance activities, retail sampling of additional dairy products and locations, studies to further characterize the relationship between different time and temperature combinations provided in the PMO, studies to characterize the thermal inactivation kinetics of this virus, and examination of levels of virus in raw, unpasteurized milk. The agency is committed to producing gold-standard science and data and will share additional results as soon as possible. The FDA will continue to work in closely partnership with USDA on many of these efforts.
Importantly, the FDA continues to emphasize its longstanding recommendations regarding the consumption of unpasteurized (raw) milk because it has the potential to be contaminated with pathogens that cause illness and it has been linked to numerous foodborne illness outbreaks in the past. Based on the limited research and information available, we do not know at this time if the HPAI H5N1 virus can be transmitted to humans through consumption of raw milk and products made from raw milk from infected cows. Pasteurization is a proven process with a 100-year history of protecting public health and is highly effective at eliminating the dangers associated with consuming raw milk.
Today, the FDA is making available an agenda that outlines various research efforts the agency is undertaking to help ensure the safety of our commercial milk supply during the outbreak of Highly Pathogenic H5N1 Avian Influenza (H5N1) virus in dairy cattle.
Our H5N1 research activities continue to follow stepwise, scientific study methods that are designed to help understand the characteristics of inactivation methods for H5N1 in dairy products, ensure the safety of retail dairy products, and mitigate the impact of this virus using a One Health strategy.
While our initial assessment of the milk safety system continues to be affirmed by sampling and testing of retail dairy products, there remain a number of collective activities being undertaken to ensure the continued effectiveness of the federal-state milk safety system. The FDA will continue to follow a sound scientific process to inform the agency’s public health decisions related to food safety.
As outlined in the FDA’s research agenda released today, the FDA is working on multiple efforts to understand the effectiveness of pasteurization and other inactivation methods. We have been actively engaged in conducting studies using continuous flow pasteurization equipment, reflective of those in commercial use, to help confirm pasteurization parameters that are effective at inactivating H5N1 HPAI virus during milk processing. These ongoing studies will provide the information to validate evidence of the effectiveness of pasteurization in ensuring that no viable HPAI H5N1 virus is present in the commercial milk supply. The agency is committing to sharing results from these ongoing studies in the near future.
The FDA’s research agenda also highlights ongoing efforts to ensure the safety of retail dairy products. Earlier this week, the agency launched a second sampling survey of dairy products available at retail locations nationwide to expand our knowledge of HPAI H5N1. This retail sampling effort is intended to address remaining geographic and product gaps from the initial sampling of the commercial milk supply that the FDA conducted between April and May of this year; results were announced in the agency’s May 10 update indicating that no viable virus was detected in 297 retail samples of milk and milk products.
The FDA’s second sampling survey is testing approximately 155 dairy products for H5N1 collected at retail locations, including fluid milk and products such as aged raw milk cheese, pasteurized milk and pasteurized cheeses, cream cheese, butter and ice cream. The samples collected include dairy products processed in states that were not included in the agency’s first phase of retail research. Additional samples are being taken from areas included in our previous survey to help provide a more representative picture based on the level of dairy product production that occurs in certain regions.
The FDA, in conjunction with our government partners, is committed to providing further updates around our research efforts and will provide results on the second phase of our ongoing retail sampling survey effort in the near future.
In our May 1, 2024 sampling update, we announced that all 297 samples from the FDA’s initial survey of retail dairy products were found to be negative for viable Highly Pathogenic H5N1 Avian Influenza (H5N1 HPAI) virus. Today, for continued transparency, the FDA is providing additional information on our retail sample survey.
The samples taken as part of this survey were collected at retail locations in 17 states by milk specialists in the FDA’s Office of Regulatory Affairs. USDA Agricultural Research Service’s U.S. National Poultry Research Center (ARS) analyzed these samples using stepwise, scientific methods. This testing included first conducting quantitative real time polymerase chain reaction (qRT-PCR) screening to determine if any of the retail samples contained H5N1 viral nucleic acid. The samples that were found to contain viral nucleic acid during qRT-PCR screening were followed with gold-standard egg inoculation testing conducted by ARS to determine if they contained live virus. None of the samples were positive for live virus. ARS scientists are currently obtaining peer review of their analysis as a first step to publishing these results. The prepublication is available at https://www.medrxiv.org/content/10.1101/2024.05.21.24307706v1.
While the FDA collected the 297 samples at retail locations in 17 states, these retail samples represent products produced at 132 processing locations in 38 states. The information in the first chart below shows the state in which the product was processed. Because the intent of our study was to assess a variety of products, samples were selected to be representative of processors in states that have been reported to have impacted dairy cattle and those that have not. Of note, the location of where milk was processed does not indicate where the milk was produced. This is because milk could be produced from cows on a farm or farms a few states away, processed (pasteurized) in a different state, and then be available for purchase in yet another state.
The charts below provide additional details on the samples taken as part of our survey of retail dairy products.
As noted previously, qRT-PCR-positive results do not necessarily represent live virus that may be a risk to consumers. Therefore, viability testing by egg inoculation was performed on the qPCR samples that were positive for viral nucleic acid. All of these samples did not detect any viable virus. If samples tested by qRT-PCR were negative, no further testing was performed since those samples did not contain HPAI viral nucleic acid. These findings further support our assessment that the milk safety system including pasteurization is effective against this virus and that the commercial milk supply remains safe.
Retail samples were collected between April 18-22 and represent a snapshot in time. This testing did not detect any live, infectious virus.
Table 1: Breakdown of Retail Sample Results by State Where Milk Was Processed
| State Where Milk Was Processed (May Not Relate to Where Milk Was Produced) | Detection of Live Virus in Retail Product(s) | Number of Retail Product Samples Tested | Retail Product Samples Negative for Viral RNA (qRT-PCR Screening -) |
Retail Product Samples Positive for Viral RNA (qRT-PCR Screening +) |
Retail Product Sample Results for Live Virus (Viability Testing by Egg Inoculation) |
|---|---|---|---|---|---|
| AR | No | 5 | 0 | 5 | 0 |
| AZ | No | 5 | 4 | 1 | 0 |
| CA | No | 21 | 21 | 0 | Not Performed (Negative qRT-PCR) |
| CO | No | 8 | 5 | 3 | 0 |
| CT | No | 2 | 2 | 0 | Not Performed (Negative qRT-PCR) |
| FL | No | 10 | 9 | 1 | 0 |
| GA | No | 8 | 8 | 0 | Not Performed (Negative qRT-PCR) |
| IA | No | 11 | 11 | 0 | Not Performed (Negative qRT-PCR) |
| ID | No | 4 | 4 | 0 | Not performed (Negative qRT-PCR) |
| IL | No | 5 | 5 | 0 | Not Performed (Negative qRT-PCR) |
| IN | No | 9 | 8 | 1 | 0 |
| KS | No | 7 | 1 | 6 | 0 |
| KY | No | 4 | 1 | 3 | 0 |
| MA | No | 4 | 4 | 0 | Not Performed (Negative qRT-PCR) |
| ME | No | 2 | 2 | 0 | Not Performed (Negative qRT-PCR) |
| MI | No | 13 | 9 | 4 | 0 |
| MN | No | 16 | 13 | 3 | 0 |
| MO | No | 10 | 7 | 3 | 0 |
| NC | No | 5 | 4 | 1 | 0 |
| ND | No | 2 | 2 | 0 | Not Performed (Negative qRT-PCR) |
| NE | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| NH | No | 1 | 1 | 0 | Not Performed (Negative qRT-PCR) |
| NJ | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| NV | No | 4 | 4 | 0 | Not Performed (Negative qRT-PCR) |
| NY | No | 38 | 38 | 0 | Not Performed (Negative qRT-PCR) |
| OH | No | 8 | 5 | 3 | 0 |
| OK | No | 12 | 2 | 10 | 0 |
| OR | No | 10 | 10 | 0 | Not Performed (Negative qRT-PCR) |
| PA | No | 2 | 2 | 0 | Not Performed (Negative qRT-PCR) |
| SC | No | 3 | 0 | 3 | 0 |
| TN | No | 3 | 3 | 0 | Not Performed (Negative qRT-PCR) |
| TX | No | 26 | 13 | 13 | 0 |
| UT | No | 5 | 5 | 0 | Not Performed (Negative qRT-PCR) |
| VA | No | 6 | 6 | 0 | Not Performed (Negative qRT-PCR) |
| VT | No | 2 | 2 | 0 | Not Performed (Negative qRT-PCR) |
| WA | No | 8 | 8 | 0 | Not Performed (Negative qRT-PCR) |
| WI | No | 11 | 11 | 0 | Not Performed (Negative qRT-PCR) |
| WV | No | 1 | 1 | 0 | Not Performed (Negative qRT-PCR) |
Table 2: Breakdown of Retail Sample Results by Product Type
| Product Category |
Number of Retail Product Samples | Detection of Live Virus in Retail Product | Retail Product Samples Negative for Viral RNA (qRT-PCR Screening -) |
Retail Product Samples Positive for Viral RNA (qRT-PCR Screening +) |
Percent of Retail Product Samples Positive for Viral RNA (via qRT-PCR screening) |
Retail Product Sample Results for Live Virus (Confirmatory Virus Culture) |
|---|---|---|---|---|---|---|
| Skim Milk | 36 | No | 32 | 4 | 11.1% | 0/4 |
| 1% Milk | 28 | No | 19 | 9 | 32.1% | 0/9 |
| 2% Milk | 58 | No | 42 | 16 | 27.6% | 0/16 |
| Whole Milk | 68 | No | 52 | 16 | 23.5% | 0/16 |
| Cottage Cheese | 21 | No | 20 | 1 | 4.8% | 0/1 |
| Cream | 17 | No | 14 | 3 | 17.6% | 0/3 |
| Half and Half | 25 | No | 19 | 6 | 24.0% | 0/6 |
| Sour Cream and Similar | 30 | No | 25 | 5 | 16.7% | 0/5 |
| Yogurt | 14 | No | 14 | 0 | 0 | NA |
| Total | 297 | None | 237 | 60 | 20.2% | 0/60 |
This retail sampling study was designed to assess the effectiveness of the PMO milk safety system; it was not designed to assess the prevalence of H5N1 in dairy herds. It is important to underscore that milk purchased for the retail study in a particular state does not mean that it was produced or processed in that state. Commercial milk is typically pooled from many dairy farms, pasteurized in bulk and distributed to a variety of states. Even if a sample was collected in one particular state, the milk in a consumer package could have come from cows on several farms located in several states, pasteurized in a different state from the states where the milk was produced, and available for purchase in yet another state.
To further validate pasteurization effectiveness against the recently identified H5N1 virus, we are undertaking a pasteurization study designed to better replicate real-world conditions. Preliminary results from this work are expected in the near future.
The FDA is announcing an additional set of results from our national commercial milk sampling study underway in coordination with USDA. The study includes 297 total retail dairy samples. New preliminary results of egg inoculation tests on a second set of 201 quantitative polymerase chain reaction (qRT-PCR)-positive retail dairy samples, including cottage cheese and sour cream, in addition to fluid milk, show that pasteurization is effective in inactivating HPAI H5N1.
This additional preliminary testing did not detect any live, infectious virus.
In addition to preliminary results released late last week on an initial set of 96 retail milk samples, these results reaffirm our assessment that the commercial milk supply is safe.
To ensure the safety of milk-derived products for our youngest populations, the FDA also tested samples of retail powdered infant formula and powdered milk products marketed as toddler formula. All qRT-PCR results of formula testing were negative, indicating no detection of HPAI H5N1 viral fragments or virus in powdered formula products so no further testing was required for these samples. The FDA is continuing to identify additional products that may be tested.
The FDA is also continuing to test samples of pooled raw milk that has been routed to pasteurization and processing for commercial use. This will be used as a basis to characterize potential virus levels that pasteurization may encounter – and will be used to inform studies to further validate pasteurization.
As this situation evolves, the FDA will continue to consider all ongoing scientific research related to the effectiveness of pasteurization for HPAI in bovine milk. We are also committed to continued surveillance of milk production, processing and pasteurization to help ensure the safety of the milk supply. Our state partners are integral to this process, and we are working with them on a continual basis. We will also continue working with our state co-regulators on managing this emerging disease.
The FDA continues to advise strongly against the consumption of raw milk and recommends that industry does not manufacture or sell raw milk or raw milk products.
The FDA has received additional results from an initial limited set of geographically targeted samples as part of its national commercial milk sampling study underway in coordination with USDA. The FDA continues to analyze this information; however, preliminary results of egg inoculation tests on quantitative polymerase chain reaction (qRT-PCR)-positive retail milk samples show that pasteurization is effective in inactivating HPAI.
This additional testing did not detect any live, infectious virus. These results reaffirm our assessment that the commercial milk supply is safe.
In addition, several samples of retail powdered infant formula were tested, as well as powdered milk products marketed as toddler formula. All qRT-PCR results of formula testing were negative, indicating no detection of viral fragments or virus in powdered formula products.
The FDA is further assessing retail samples from its study of 297 samples of retail dairy products from 38 states. All samples with a PCR positive result are going through egg inoculation tests, a gold-standard for determining if infectious virus is present. These important efforts are ongoing, and we are committed to sharing additional testing results as soon as possible. Subsequent results will help us to further review our assessment that pasteurization is effective against this virus and the commercial milk supply is safe.
Epidemiological signals from our CDC partners continue to show no uptick of human cases of flu and no cases of HPAI H5N1, specifically, beyond the one known case related to direct contact with infected cattle.
Today, the FDA received some initial results from its nationally representative commercial milk sampling study. The agency continues to analyze this information; however, the initial results show about 1 in 5 of the retail samples tested are quantitative polymerase chain reaction (qRT-PCR)-positive for HPAI viral fragments, with a greater proportion of positive results coming from milk in areas with infected herds. As previously noted and outlined in our summary below, qRT-PCR-positive results do not necessarily represent actual virus that may be a risk to consumers. Additional testing is required to determine whether intact pathogen is still present and if it remains infectious, which would help inform a determination of whether there is any risk of illness associated with consuming the product. The FDA is further assessing any positive findings through egg inoculation tests, a gold-standard for determining if infectious virus is present. Early work by NIH-funded investigators indicates an absence of infectious virus in their studies of retail milk. To date, the retail milk studies have shown no results that would change our assessment that the commercial milk supply is safe. Epidemiological signals from our CDC partners continue to show no uptick of human cases of flu and no cases of HPAI H5N1, specifically, beyond the one known case related to direct contact with infected cattle. These important efforts are ongoing, and we are committed to sharing results from both the qRT-PCR and egg inoculation tests as soon as possible.
Multiple tests are used to assess the safety of food items. Understanding how and why different methodologies are used and work, as well as how results fit into the larger picture, is critical to interpret any findings.
- Quantitative polymerase chain reaction (qRT-PCR) is a screening tool used to determine the presence or absence of an organism’s genetic material in a sample. A positive qRT-PCR means that the genetic material from the targeted pathogen was detected in the sample, but that does not mean that the sample contains an intact, infectious pathogen. That’s because qRT-PCR tests will also detect the residual genetic material from pathogens killed by heat, like pasteurization, or other food safety treatments. Importantly, additional testing is required to determine whether intact pathogen is still present and if it remains infectious, which determines whether there is any risk of illness associated with consuming the product.
- Embryonated Egg Viability Studies are considered the “gold standard” for sensitive detection of active, infectious virus. These studies are one of the types of additional tests necessary following PCR testing. These studies are done by injecting an embryonated chicken egg with a sample and then evaluating to see whether any active virus replicates. While this provides the most sensitive results, it takes a longer time to complete than other methods.
- Madin-Darby Canine Kidney (MDCK) Cell Culture is different type of additional test used following PCR testing to detect live, infectious virus. This is done by injecting a sample into specific tissue cells to determine whether any live virus is present and replicates. This method can usually be done more quickly than embryonated egg viability studies, but it is not as sensitive and may provide false negative results when the amount of virus in the sample is very low.
- HPAI in Livestock (USDA)
- Dear Veterinarian Letter Regarding Use of Aspirin Products in Lactating Dairy Cattle (FDA)
- H5 Bird Flu: Current Situation (CDC)
- How CDC is monitoring influenza data among people to better understand the current avian influenza A (H5N1) situation (CDC)
Additional Information