Protein Surveillance Assignment (PSA) Summary Report
Executive Summary
Background
Scope
Conclusion
Executive Summary
The Protein Surveillance Assignment (PSA) is the latest food defense related FDA field activity. The PSA was designed as a proactive effort to review protein sources being imported and in response to the investigations of the pet deaths in the United States (U.S.) that were associated with the consumption of pet food contaminated with melamine, cyanuric acid, ammelide, and ammeline. The Food and Drug Administration (FDA) noted that the same protein sources being used for pet foods could also be used as protein sources for human food. Thus, the FDA took this proactive measure to help ensure the safety of the U.S. food and feed supply. This was the first food defense related FDA field activity that concurrently looked at both the food and feed supply. It was planned and conducted jointly with several FDA operational divisions (Center for Food Safety and Applied Nutrition (CFSAN), Center for Veterinary Medicine (CVM), Office of Regulatory Affairs (ORA)), as well as the Food Emergency Response Network (FERN) Laboratories. PSA activities began on May 1, 2007 and continued until June 30, 2007. The assignment focused on imported vegetable protein extracts and finished food and feed products in domestic status. PSA activities ran concurrently with the pet food field investigations and ORA's Prior Notice Center (PNC) issuing additional directed assignments for both ingredients/products of interest in import status based on Import Alert #99-29 as well as incoming imports of those products identified in this assignment.
The over-arching goals of this assignment were to:
- Examine, through inspection and sample analysis, ingredients and finished products imported from China (or transshipped from China) for the presence of melamine, cyanuric acid, ammelide, and ammeline ;
- Launch an educational campaign to make the food and feed industry more aware of the issues in light of the recent pet food recalls involving wheat gluten, rice protein concentrate and corn gluten;
- Deter intentional contamination of food and feed through heightened and targeted preventive activities at various points in the chain of supply;
The PSA was originally scheduled to run for thirty days but activities were continued for another thirty days in order to gain additional confidence in the safety of the human food supply. During the PSA, FDA's field personnel were tasked with conducting records examinations and inspections as well as collecting and analyzing domestic and import samples for the presence of melamine, cyanuric acid, ammelide, and ammeline. Throughout the duration of the assignment, over 200 inspections were made throughout the country and over 220 samples were analyzed.
Background
In March 2007, FDA began investigating pet deaths in the U.S. associated with the consumption of pet food contaminated with melamine. Shipments of both wheat gluten and rice protein imported from China were implicated as part of that 2007 investigation. In addition, corn gluten contaminated with melamine used in pet foods was implicated during the same period with dog illnesses and deaths in South Africa. At that time (and since that time) there was no indication that the findings in pet food were part of a deliberate attack on the U.S. food supply, nor were there any indications that melamine had been added to ingredients other than those used in the pet foods. However, FDA believed t hat in the interest of being proactive and raising awareness it was important for the FDA to conduct an assignment looking at a wider scope of domestic and imported products and to complement other actions being undertaken through the pet food field investigations and the Import Alert put in place by ORA's Division of Import Operations.
Scope
The PSA provided an opportunity for multiple components of the FDA and the Food Emergency Response Network (FERN) laboratories to work together in a proactive approach to ensure the safety of the U.S. food and feed supply. The planning group (CFSAN, CVM, ORA, and FERN laboratories) worked together to make decisions and develop a strategy for the assignment. The assignment focused on tiering imported food and feed commodities to determine where the greatest risk for adulteration might lie and then effectively implementing a plan to investigate and sample those commodities. The criteria for tiering included: country of origin, protein content and intended populations. The planning group developed an eight week schedule to examine products of interest which included both imported ingredients as well as food and feed products already in the U.S. Additionally, representatives from CFSAN, CVM and ORA modified the targeting rules employed by PNC to identify suspect products of interest. For example, the product codes targeted in the PSA were included in the targeting rules, as well as products that may have been transshipped from China.
The PSA included information on specific firms that received or produced the products of interest. FDA inspectors were asked to visit these firms and complete the following tasks:
- Conduct food safety inspections
- Disseminate and discuss ALERT food defense awareness information
- Collect and submit trace back/trace forward information for the products of interest
- Collect a sample of the products of interest and send to a participating FERN lab for analysis
A critical component of the PSA was the ability of the FERN Laboratories to:
- Identify and/or validate methods and ensure matrix compatibility for analysis of melamine, cyanuric acid, ammelide, and ammeline
- Conduct physical sample analysis and screen for other contaminants
- Complete analysis and report results within two working days
| Product Codes | Examples of products that were identified as products of interest for the Protein Surveillance Assignment |
|---|---|
| Whole Grain/ Milled Grain Products/ Starch- 02 | Soybeans, whole grain, wheat flour, rice flour, soybean flour, soybean meal, soybean powder, rice, corn (whole or grain), wheat germ, wheat gluten, wheat meal, |
| Milk/ Butter/ Dried Milk Products- 09 | Acid (lactic) casein |
| Vegetable Protein Products- 18 | Soybean protein powder, soy fiber, wheat protein, cereal base meat extenders |
| Gelatin/ Rennet/ Pudding Mix/ Pie Filling- 35 | Agar (Gelatin Product), Pudding (Pie Filling) Mix, Gelatin, Plain |
| Baby Food Products- 40 | Milk Base Formula Product, Infant formula pre-mix bulk product |
| Dietary Conventional Food/ Meal Replacements- 41 | Foods with Supplemental Nutrients Added, with or without artificial sweeteners, Dietary Conventional Foods, Foods (including Water) with Excessive Nutritional Claims, Nutritionally Complete Formulations, Meal Replacements (Not labeled for treatment of disease), Medical foods |
| Vitamin/ Mineral/ Protein/ Unconventional Diet (Human/Animal)-54 | Vitamin, Mineral, Proteins and Unconventional Dietary Specialties For Humans and Animals, protein |
| Animal Feed (Non-Medicated)- 70 | Soybeans, Soybean Meal Whole/Ground Grains Animal, Animal feeds, Wheat Whole/Ground Grains Animal, Mixed Feed Ration For Animals, Domestic Aquaculture |
| Byproducts for Animal Foods- 71 | Rice Mill byproduct, Oilseed byproduct, poultry byproduct, marine byproduct, dairy byproduct |
Goals & Accomplishments
The PSA utilized a proactive approach for FDA to examine ingredients and finished products imported from China (or transshipped from China) for the presence of melamine, cyanuric acid, ammelide, and ammeline. FDA investigators were able to visit over 200 different firms throughout the United States within the time period of this specialized assignment and make members of the food and feed industry more aware of adulteration issues through the dissemination of the ALERT messages. Inspections were performed at various points in the supply chain including, importers, warehouses, and manufacturers. Samples were collected primarily during visits to domestic food manufacturers or in the case of imports, at the point of entry.
FERN laboratories were able to develop the analytical methods used to detect melamine, cyanuric acid, ammelide, and ammeline. These methods were made available to the public and FDA encouraged industry to use in their existing analytical testing.
Eight nationwide FERN Laboratories were able to analyze over 220 samples of different products of which four were found to be positive. The four positive samples were found to be below the level of concern and unlikely to pose a human health risk based on the Interim Melamine and Analogues Safety/Risk Assessment. All of the laboratory results were analyzed and reported with the established timeframes.
The PNC targeted and directed activities related to imported shipments of the products of interest from China, and also identified those that may have been potentially transshipped from China through other countries. Those targeted shipments deemed as posing the highest risk were directed for examination and sampling. In addition, the PNC identified domestic firms involved in the subject high risk import shipments that were previously unknown or not previously inspected by FDA. These firms were referred for follow up inspection. The PNC also served as the liaison point with CBP to communicate actions and activities they conducted under their own gluten assignment. This coordination was completed in order to prevent a duplication of FDA and CBP efforts. During the PSA, the PNC issued forty nine (49) directed import examination/sample collection assignments of various products including wheat gluten/flour products, Soy bean products, finished pet food/biscuits, canned fried gluten, corn gluten products, ovine meal, fish meal, soy milk, casein, wheat cakes, and rice flour. The assignments involved imported products from various countries including China, Taiwan, Canada, France, Netherlands, New Zealand, Mexico, Germany, and Poland.
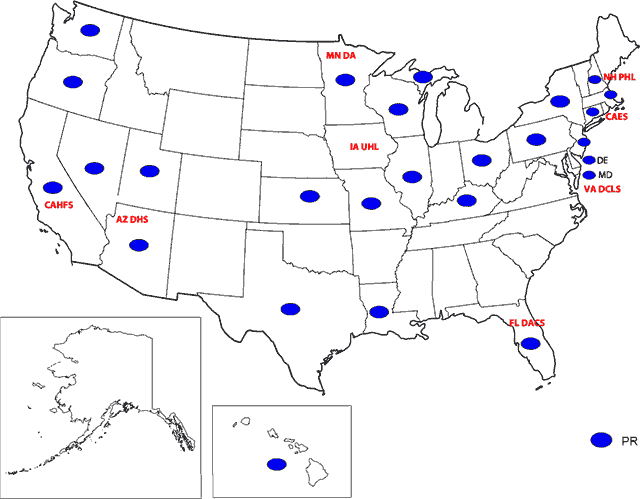
Map: States Where Inspections Were Performed As Part of the PSA & Participating FERN Labs
Conclusion
The 2007 PSA was undertaken by the FDA to ensure that the melamine, cyanuric acid, ammelide, and ammeline contamination found in pet food was not a more widespread problem in the food and feed system. Throughout the duration of the assignment, over 200 inspections were made throughout the country and over 220 samples were analyzed. This complementary component was run in tandem with both the pet food field investigations and the PNC directed activities; demonstrating the ability to pair a proactive effort with an outbreak intervention.
The PSA served as the latest opportunity to enhance coordination, communication and reporting among the different components of the FDA and the FERN laboratories. The planning group built upon previous food defense field assignment models and lessons learned to create and lead an efficient and effective response to the adulteration concern. Additionally, during visits to the firms, FDA focused on prevention practices by reminding members of the food industry about the importance of knowing your suppliers, packaging operators, and contract manufacturers; a key point highlighted in the ALERT message. The FERN Laboratories ensured that adequate methods were available and accurate results were provided in a timely manner. Overall, the PSA demonstrated the collaborative ability and willingness of all participants to respond to an identified food safety concern.
Description of Map: States Where Inspections Were Performed As Part of the PSA & Participating FERN Labs
Map of the United States and its territories depicting states where inspections were performed and the Food Emergency Response Network (FERN) Laboratories that participated in the Protein Surveillance Assignment (PSA). As part of the PSA, inspections were performed in the states of Washington, Oregon, California, Nevada, Arizona, Utah, Texas, Kansas, Louisiana, Missouri, Minnesota, Wisconsin, Michigan, Illinois, Kentucky, Ohio, Pennsylvania, Florida, New York, New Hampshire, Massachusetts, Connecticut, New Jersey, Delaware, Maryland, Hawaii and Puerto Rico. All other states and territories have not adopted the 1999 FDA Food Code. Participating FERN Laboratories were; Arizona Department o f Health Services (AZ DHS), California Animal Health & Food Safety Laboratory (CAHFS), Minnesota Department of Agriculture (MN DA), Iowa University Hygienic Laboratory (IA UHL), Florida Department of Agriculture and Consumer Services (FL DACS), Virginia Division of Consolidate Laboratory Services (VA DCLS), Connecticut Agricultural Experiment station (CAES), and New Hampshire Public Health Laboratory (NHPHL).