Accredited Third-Party Certification Program
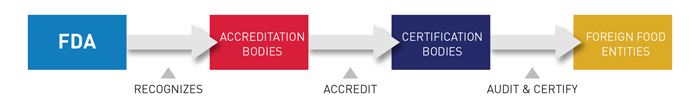
Accredited Third-Party Certification is a voluntary program in which FDA recognizes “accreditation bodies” that will have the responsibility of accrediting third-party “certification bodies.” The certification bodies will conduct food safety audits and issue certifications of foreign food facilities.
Program Application
- Apply to become an Accreditation Body
- Select "Create New Account" and then select the appropriate box under FSM
- Accreditation Body Electronic Portal User Instructions (PDF: 6MB)
- Certification Body Electronic Portal User Instructions (PDF: 6.48MB)
- Key Facts About the Program (PDF: 79KB)
- Public Registry of Recognized Accreditation Bodies
- Public Registry of Accredited Third-Party Certification Bodies
- User Fees
These certifications are used for two purposes.
- Certifications can establish eligibility for participation in the Voluntary Qualified Importer Program (VQIP), which offers expedited review and entry of food.
- In rare and specific circumstances FDA can require that an imported product be certified to prevent a potentially harmful food from entering the U.S.
The Agency’s imported food goal is twofold:
- To address potential safety issues before the food reaches the United States; and
- To help ensure that imported foods are produced in accordance with the same safety standards as those required of U.S. foods.
Accreditation bodies under the Accredited Third-Party Certification Program must:
- Assess third-party certification bodies to determine if they can be accredited. This includes observing a representative sample of the applicant’s work;
- Monitor the performance of the certification bodies it accredits. Accreditation bodies must notify FDA of any change in, or withdrawal of, accreditations it has granted;
- Assess and correct any problems in the accreditation body’s own performance;
- Submit monitoring and self-assessment reports and other notifications to FDA;
- Maintain and provide FDA with access to the records that the program requires.
Accreditation bodies may begin to accredit certification bodies to issue certifications under this program once they receive recognition from FDA.
- In January 2018, FDA announced ANSI-ASQ National Accreditation Board as the first recognized accreditation body under the Accredited Third-Party Certification Program.
Related Guidance Documents
- Draft Guidance for Industry: The Accredited Third-Party Certification Program: Questions and Answers
- Guidance for Industry and FDA Staff: Accreditation of Third-Party Certification Bodies to Conduct Food Safety Audits to Issue Certifications: Model Accreditation Standards
- Guidance for Industry: FDA's Voluntary Qualified Importer Program
Background
- Accredited Third-Party Certification Rule
- Constituent Update: FDA Takes Important New Steps to Strengthen Oversight of Food Imports
Additional Information
- Industry Resources on Third-Party Audit Standards and FSMA Supplier Verification Requirements
- Accredited Third-Party Certification Program Voluntary Audit Templates
- Questions & Answers
Contact Us
- How to Contact FDA about FSMA (Technical Assistance Network)
Voluntary Graphic Element & QR Code
A TPP program QR code is available to assist FDA and stakeholders with communicating the status of participants in the TPP program.
TPP graphic elements are available to help increase awareness of the TPP program.
Download these materials and read these guidelines for using them by visiting Voluntary Graphic Element & QR Code Guidelines.