How Does FDA Prioritize Domestic Human Food Facility Inspections?
Inspections to Protect the Food Supply
The FDA Food Safety Modernization Act (FSMA) mandates inspection of domestic food facilities that are required to register with the FDA* at a frequency that is based on a facility’s risk level. High-risk facilities are required to be inspected at least once every 3 years and non-high-risk facilities are required to be inspected at least once every 5 years. These time frames represent the minimum inspection frequency requirements; however, facilities may be inspected more frequently if necessary to protect public health. Additionally, it’s important to note that the FDA Reauthorization Act (FDORA) now requires infant formula facilities to be inspected annually which the FDA considers in its work planning (see the last question below).
This webpage will specifically address how the FDA prioritizes domestic human food facility inspections under FSMA.
*The Bioterrorism Act of 2002 requires domestic and foreign facilities that manufacture, process, pack, or hold food for human or animal consumption in the United States to register with the FDA unless an exemption applies.
Which facilities are subject to the FSMA inspection frequency mandate?
The FSMA inspection frequency mandate applies to domestic food facilities that are required to register with the FDA.
In addition to the FDA’s Food Facility Registration Database, the agency also uses the Official Establishment Inventory (or OEI). The OEI includes some facilities whose registrations expired when the facilities failed to complete their required biennial registration renewal, and other facilities which have never registered. Using the OEI in conjunction with the Food Facility Registration Database helps ensure that facilities which have never registered or facilities with lapsed registrations are still inspected.
What risk factors does the FDA evaluate when determining if a facility is high-risk?
FSMA mandated the identification of high-risk facilities based on known safety risk factors. The FSMA inventory currently contains roughly 72,000 domestic human food facilities, with approximately 22% categorized as high-risk and 78% categorized as non-high risk.
The following table outlines the risk factors, as promulgated by FSMA and stated in the Federal Food, Drug, and Cosmetic Act (FD&C Act), and the corresponding data we assess to evaluate those risk factors.
Table 1. Risk Factors and Supporting Data
| Known Safety Risk Factors (per section 421(a)(1) of the FD&C Act) | Supporting Data |
|---|---|
| Known safety risks of the food | Facilities manufacturing, processing, packing, or holding food in commodity categories associated with high incidences of: Class I recalls Outbreaks of foodborne illness Violative samples (laboratory class 3) Inspections classified as Official Action Indicator (OAI) |
| Compliance history of a facility | Facilities with a history of: Class I or II recalls Outbreaks of foodborne illness Violative samples (laboratory class 3) Inspections classified as OAI Compliance actions taken Inspections with no significant violations classified “No Action Indicated” (As opposed to an OAI inspection which can increase the risk-profile, an NAI classification could indicate less risk, which will be factored into our evaluation) |
| Facility’s hazard analysis and risk-based preventive controls | FDA has improved information technology systems that support FDA inspection reporting to monitor preventive controls inspection outcomes. FDA utilized the new information in FSMA performance measures and is further examining its use to support this FSMA risk factor. |
| Priority under section 801(h)(1) of the FD&C Act (inspections of food offered for import, especially to detect intentionally adulterated food) | The FDA is further examining the use of available data to support this risk factor for domestic human food facilities. |
| Certification per section 801(q) or section 806 of the FD&C Act (certification of certain imported foods and importers who participate in the voluntary qualified importer program (VQIP)) | The FDA is further examining the use of available data to support this risk factor for domestic human food facilities. |
| Any other criteria deemed necessary | Type of Activity (establishment type). |
How does the FDA use these factors to evaluate risk and prioritize facilities for inspection?
FDA’s approach to risk ranking has evolved over time to leverage new data as we continue to improve IT systems and automated processes to better assess the FSMA inventory against the FSMA risk factors. In 2017, the agency launched an automated Human Food Facility Risk Categorization tool that assesses the risk factors against the FSMA inventory every two weeks. The tool allows the FDA to continuously evaluate risk as new facilities enter the market, existing facilities go out of business, or their compliance history changes.
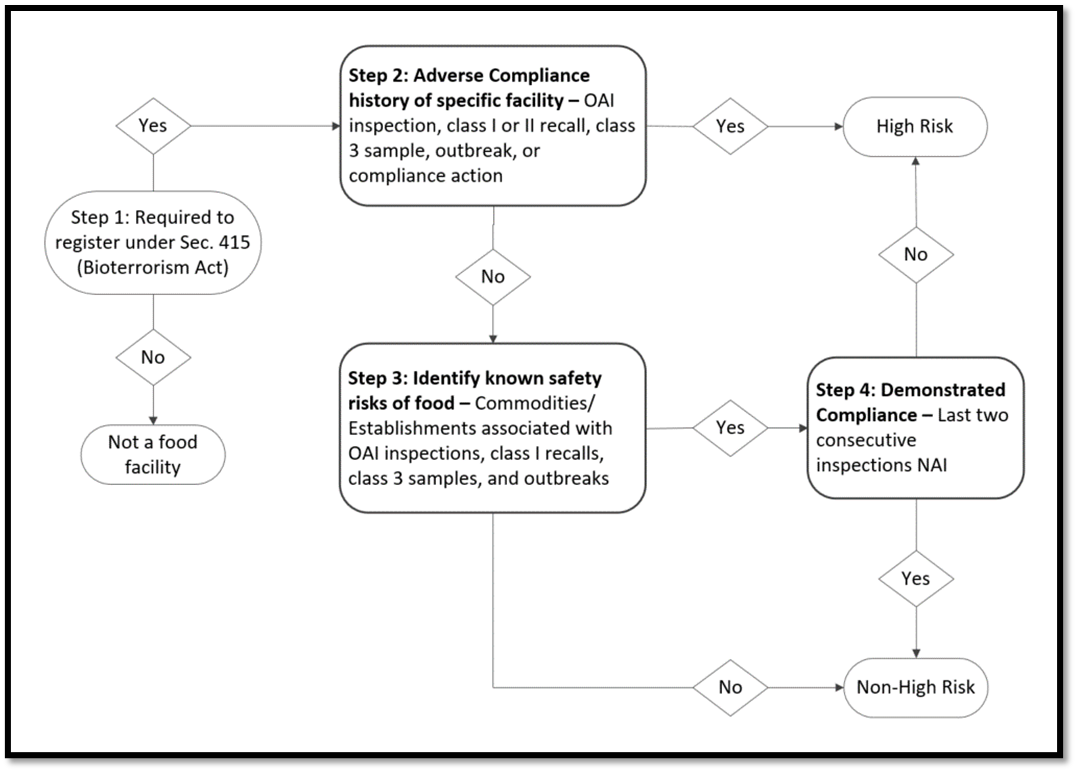
The summary diagram, Domestic Human Food Facility Risk Categorization, illustrates how the FDA determines if a facility is high-risk.
Figure 1. Domestic Human Food Facility Risk Categorization
Step 1: Determine if a facility is required to register under Section 415 of the FD&C Act. If no, the establishment is not subject to the risk-based inspection frequency mandates. If yes, move to Step 2.
Step 2: Determine if the facility’s compliance history includes an OAI or NAI inspection, Class I or II recall, laboratory class 3 sample, outbreak, or compliance action within the previous 5 years. If yes, the facility is determined to be high-risk. If no, move to Step 3.
Step 3: Determine if the facility manufactures, packs, processes, or holds a food with known food safety risks, such as foods associated with OAI inspections, Class I recalls, laboratory class 3 samples, and/or outbreaks. If no, the facility is determined to be non-high-risk. If yes, move to Step 4.
Step 4: Determine if the facility’s compliance history includes two consecutive NAI inspections as the most recent inspections reported within the previous 6 years. If no, the facility is determined to be high-risk. If yes, the facility is determined to be non-high-risk
The FDA collaborates with state agencies to enhance compliance oversight through inspectional partnerships. State-conducted inspections, especially under FDA contracts and partnerships, help the FDA meet inspection frequency mandates and assist in comprehensive coverage of regulated facilities.
What additional considerations might affect the frequency of inspections?
FSMA sets the minimum frequency for domestic food facility inspections; however, facilities may be inspected more often by FDA and state partners. More frequent inspections may be conducted based on our risk modeling indicated in Table 1. In addition, we may have additional statutory mandates, such as the requirement for annual inspections of infant formula manufacturing facilities, as promulgated by the Food and Drug Omnibus Reform Act of 2022. We may also determine that more frequent inspections are necessary to respond to emerging public health information, to follow up on violative inspections and samples, or to conduct surveillance studies directed to particular industries.