Rapid Response Teams (RRTs) for Human and Animal Foods
Rapid Response Teams (RRTs) are multi-agency, multi-disciplinary teams that operate using Incident Command System (ICS)/National Incident Management System (NIMS) principles and a Unified Command structure to respond to human and animal food emergencies.
The desired outcome of RRT development is to minimize the time between agency notification of a human or animal food contamination event and implementation of effective control measures. To accomplish this, RRTs develop and maintain processes to:
- Prepare for and effectively respond to foodborne illness outbreaks and other food emergencies.
- Enhance intra-agency and interagency collaboration and communication.
- Jointly train and exercise staff to be ready to respond to events when they occur.
- Identify potential preventive practices to reduce foodborne illness and injury.
- Establish national best practices and tools that can be shared with other states to improve their response to food emergencies.
FDA provides multi-year cooperative agreements to states to form and maintain RRTs. These cooperative agreements require RRTs to engage partners across disciplines and jurisdictions to build core capabilities and explore innovative approaches to response.
Factsheet (Version November 2023)
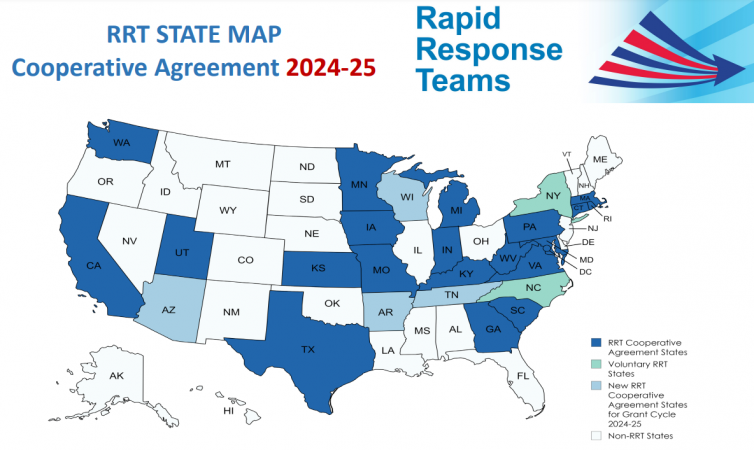
States with RRTs
There are 24 FDA funded Rapid Response Team cooperative agreement states: AR, AZ, CA, CT, GA, IA, IN, KS, KY, MA, MD, MI, MN, MO, PA, RI, SC, TN, TX, UT, VA, WA, WI, WV.
NC and NY participate in a voluntary, non-funded capacity.
There is over $5.6M in funding under this cooperative agreement program.
The RRT Best Practices Manual
The RRT Best Practices Manual features tools that can be used by programs to improve key areas of response such as communication, traceback and traceforward, laboratory analysis, and joint investigations and inspections. It also establishes metrics for rapid response capabilities that allow RRTs to assess their status, identify improvement plans, and quantify accomplishments and impact.
Download the RRT Best Practices Manual (2017 Edition)
A companion document to the RRT Best Practices Manual is the RRT Capacity Building and Mentorship Framework. This document provides a three-phase framework for incremental RRT capacity building and can be applied by any State/Division-District wishing to establish a RRT with functional rapid response capabilities aligned with the RRT Best Practices Manual and the NIMS preparedness cycle.
Download the RRT Capacity Building and Mentorship Framework
- FDA and States use Rapid Response Teams Approach to Combat Foodborne Illness through Domestic Mutual Reliance (Food Safety Magazine, April 2021)
- Meeting summary and posters from the 2020 RRT Computer-to-Computer (C2C) and 2019 RRT Face-to-Face (F2F) meeting (Association of Food and Drug Officials)
- All hands on deck: Changing roles around COVID Response (Washington State Department of Agriculture Blog, March 2021)
- Virginia’s Collaborative Investigation Cracks the Case (CDC OutbreakNet Enhanced Success Story, January 2021)
- Outbreak Investigation of Scombrotoxin Fish Poisoning Illnesses in the United States Linked to Yellowfin Tuna Imported from Vietnam – 2019 (Journal of Food Protection, January 2021)
- Lessons Learned from a Decade of Investigations of Shiga Toxin–Producing Escherichia coli Outbreaks Linked to Leafy Greens, United States and Canada (Emerging Infectious Diseases, October 2020).
- Outbreak of Norovirus Gastroenteritis Associated With Ice Cream Contaminated by Frozen Raspberries From China—Minnesota, United States, 2016 (Clinical Infectious Diseases, June 2020)
- Successful Collaboration Protects Rhode Island Residents from Foodborne Illness (APHL Success Story, May 2020)
- Listeria monocytogenes Occurrence and Adherence to Recommendations: Small and Large Retail Delicatessens in Iowa (Food Protection Trends, September 2020)
- Virginia Hepatitis A Pandora Radio Campaign A Success Story of Interagency Collaboration (AFDO Blog Post, September 2019)
- Overview of Leafy Greens - Related Food Safety Incidents with a California Link (Journal of Food Protection, March 2019)
- RRT Testimonials - Reflections on 10 Years of RRTs (2008 - 2018) (March 2019)
- Responding to Harvey and Irma: Rapid Response Teams Take Action (Food Safety Magazine April/May 2018 Issue)
- Local Rapid Response Team Deters Deliberate Food Poisoning (NACCHO Blog Post September 2017)
- Response to animal feed contamination quick, coordinated (WSDA Blog Post September 2017)
- Multistate outbreak of Listeria monocytogenes infections linked to whole apples used in commercially produced, prepackaged caramel apples: United States, 2014-2015. (Epidemiology and Infection Journal, January 2017)
- Whole-Genome Sequencing Detection of Ongoing Listeria Contamination at a Restaurant, Rhode Island, USA, 2014 (Emerging Infectious Diseases Journal, August 2016)
- Stopping Listeria required an arsenal of tools and an army of experts (APHL Blog post June 2016)
- Regional investigation of a cyclosporiasis outbreak linked to imported romaine lettuce – Nebraska and Iowa, June–August 2013 (Journal of Epidemiology and Infection, July 2016)
- Outbreak of Foodborne Botulism Associated with Improperly Jarred Pesto — Ohio and California, 2014 (MMWR Article, February 2016)
- Outbreak of Listeria monocytogenes infections linked to a pasteurized ice cream product served to hospitalized patients (Journal of Epidemiology and Infection, December 2015)
- Where the Rubber Meets the Road: RRTs in Action (Food Safety Magazine Aug/Sep 2015 issue)
- Creating the Rapid Response Road Map: Collaboration Points the Way Forward (Food Safety Magazine Oct/Nov 2015 issue)
- Outbreak of Salmonella enterica serotype Infantis infection in humans linked to dry dog food in the United States and Canada, 2012 (March 2014, Journal of the American Veterinary Medical Association)
- Use of Global Trade Item Numbers in the Investigation of a Salmonella Newport Outbreak Associated with Blueberries in Minnesota, 2010 (May 2013 Journal of Food Protection)
- Use of traceback methods to confirm the source of a multistate Escherichia coli 0157:H7 outbreak due to in-shell hazelnuts (February 2012, Journal of Food Protection)
- CalFERT Environmental Investigation Reports (2012-present)