Microbiological Surveillance Sampling: FY22-23 On-Farm Inspections and Sampling of Leafy Greens Grown in the Salinas Valley, CA, Region
Microbiological Surveillance Sampling Main Page
Seeking to gain insight into foodborne illness outbreaks involving leafy greens, the U.S. Food and Drug Administration (FDA) and the California Department of Food and Agriculture (CDFA) conducted coordinated sampling and inspections at 14 farms in the Salinas Valley, CA, agricultural region during the 2022 harvest season. CDFA conducted the inspections, and the FDA conducted the sampling.
The FDA selected the farms from the results of unresolved traceback investigations[1] from 2020 to 2021, with the goal of ascertaining the source(s) of the outbreaks, if possible. Additionally, the objectives of the agencies’ 2022 inspections and sampling in the Salinas Valley agricultural region were to identify potential contamination of leafy greens and to prevent contaminated or potentially contaminated product from entering commerce. During these field activities, conducted from July to October of 2022, the two agencies also continued their efforts to better educate growers to help them adhere to the FDA’s Produce Safety Rule.
CDFA performed the inspections under the FDA-State Produce Safety Implementation Cooperative Agreement Program, a partnership established to provide technical and financial assistance to state and territorial agencies to develop and implement produce safety programs and to help ensure compliance with the Produce Safety Rule.
All samples collected during the 2022 work in the Salinas Valley region were tested for E. coli O157:H7, a type of Shiga toxin-producing E. coli (STEC), and Salmonella spp., both of which can cause severe illness. This work was a part of the FDA’s Leafy Greens STEC Action Plan, an ongoing preventive effort to help ensure the microbiological safety of leafy greens in the United States.
Background
Outbreaks of foodborne illness involving leafy greens linked to or potentially linked to the Salinas Valley region have continued to occur, with at least one such outbreak occurring in each of the past five years.[2] The Salinas Valley region produces roughly 70 percent of the lettuce grown in California.[3] Leafy greens are among the most commonly consumed vegetables in the American diet and are typically eaten without undergoing a ‘kill step,’ such as cooking, to reduce or eliminate pathogens.
In 2021, consistent with the FDA’s prevention efforts, the agency conducted an assignment to collect lettuce from commercial cooling operations that service the Salinas Valley region to test for the presence of E. coli O157:H7 and Salmonella spp. The FDA detected non-O157 STEC and Salmonella during its 2021 assignment. Those findings, considered alongside the outbreaks, indicated to the FDA that further surveillance was warranted, but more focused on farms potentially linked to the outbreaks.
As a next step, the FDA planned inspections and sampling of farms[4] identified in unresolved traceback investigations from 2020 to 2021 as potentially associated with outbreaks of foodborne illness wherein leafy greens were the likely or suspect food vehicle. The two complementary activities, conducted during the Salinas Valley leafy greens harvest season, were envisioned to provide more complete insights into the microbiological risks to leafy greens at the identified sites.
From July to October 2022, CDFA performed inspections at nine farms of the 14 identified for this surveillance effort. As to the five farms that were not inspected, these growing locations were no longer conducting activities covered under the Produce Safety Rule on leafy greens at the time of their inspection.
CDFA’s inspectors focused on conditions and practices covered by the Produce Safety Rule at the farms. “Conditions” generally refers to field upkeep, worker health and hygiene, personnel qualifications and training, activity of domesticated and wild animals, and cleanliness of equipment and tools. “Practices” generally refers to growing, harvesting and post-harvest handling of crops, and equipment cleaning and sanitizing.
CDFA classified six of the inspectional outcomes as “voluntary action indicated,” or VAI, and the remainder as “no action indicated,” or NAI.[5] As to the VAI classifications, CDFA’s observations included inattention to bird activity, inadequate sanitation, record keeping deficiencies, and employee retraining needs. All the farm operators took prompt voluntary corrective action in response to CDFA’s observations.
The FDA obtained samples of leafy greens directly from farm fields prior to harvest. Sampling prior to harvest can provide a valuable data point related to pre-harvest conditions and minimize product loss. Farm operators generally hold their product pending their receipt of sample test results by the FDA and have voiced that post-harvest sampling can lead to loss of product. In this case, the test results were available before the fields were scheduled to be harvested, meaning there was no occasion to hold product and leafy greens associated with sampled lots would not have entered commerce, forestalling the need to conduct recalls.
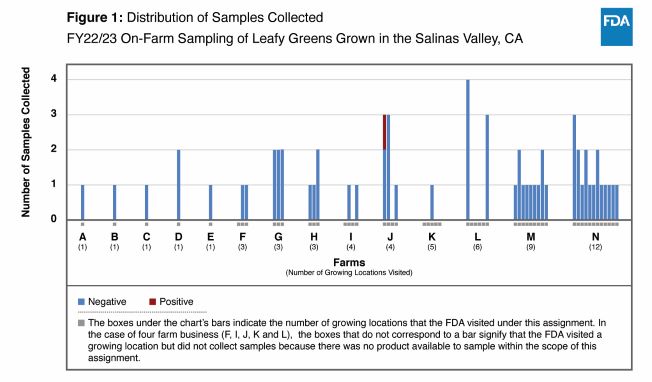
The FDA’s sampling centered on 14 farm businesses in the Salinas Valley region. In all, the agency collected and tested 63 samples (62 samples of leafy greens, and one environmental sample). The product samples consisted primarily of lettuce (i.e., iceberg, leaf and romaine), and of spinach. Each leafy greens sample was made up of 10 subsamples, and each subsample weighed about 400 grams (a little less than a pound). Most of the samples of leafy greens were untrimmed heads of lettuce. The environmental sample was a sediment sample.
All of the samples were collected from farm fields. The FDA sought to have farm personnel collect the samples using farm procedures so they could be obtained consistent with the sampling practices of each farm. When farm personnel collected the specimens, the FDA investigators observed the sampling. Of the 62 samples of leafy greens, FDA staff collected 41 samples, and farm personnel collected 21 samples.
The FDA tested all the samples for both of the target pathogens. In testing for Salmonella spp., the FDA used an polymerase chain reaction (PCR) screening assay to detect for the presence of the bacteria, followed by the agency’s Bacteriological Analytical Manual (BAM) Chapter 5 method for isolation and confirmation of samples with positive screen results. In testing for E. coli O157:H7, the FDA used the BAM Chapter 4A method, which includes an PCR assay that is specific for stx1, stx2 and E.coli O157 serotype genes, followed by cultural confirmation of isolates from samples that indicated the presence of E. coli O157:H7 during the PCR screen.
The FDA committed to notify each farm of the test results within three days of sample collection in the event of negatives and/or instances of preliminary indication,[6] and within one week of sample collection in cases of confirmed results. The FDA met its notification intervals in all instances.
Of the 62 samples of leafy greens, the FDA detected Salmonella Enteritidis in one sample of romaine lettuce. The agency performed whole genome sequencing analysis on the Salmonella recovered from the positive sample and found the bacteria to be closely related to clinical and chicken isolates. However, the FDA did not have sufficient epidemiological evidence to link the farm at which the sample was collected to clinical illnesses. The sediment sample was negative for each of the target pathogens.
The chart that accompanies this report provides the sample distribution across the 14 farm businesses visited by the FDA for purposes of the sampling (Figure 1). All farms have been de-identified within the chart.
As to the Salmonella-positive sample, operators of the farm did not harvest the crop from the area of the field considered to be represented by the sample. The FDA provided the farm management with guidance on testing protocols for microbial hazards in leafy greens and shared its findings with CDFA.[7]
This surveillance effort and the related follow-up actions prevented contaminated leafy greens from entering commerce but did not find additional evidence linking any of the 14 farms to the foodborne illness outbreaks in 2020 and 2021. These results add to the evidence that sources and routes of pathogenic contamination on leafy greens are very difficult to discern and that sampling and retrospective investigations, alone, are not sufficient to glean the root causes of outbreaks linked to leafy greens. The FDA will continue to explore new ways to investigate the root causes of outbreaks linked to leafy greens and will continue its work with industry, academia and state regulators to prevent outbreaks and strengthen leafy greens’ microbiological safety.
Among the FDA’s efforts in recent years, the Leafy Greens STEC Action Plan is a collaborative and multi-faceted approach to enhancing the safety of leafy greens. The plan includes prioritized inspections, focused sampling, stakeholder engagement, data sharing, root cause investigations, and advancements in the science of detection and prevention.
Consistent with the plan, the FDA seeks ways to gain meaningful and timely information on potential routes and sources of contamination on leafy greens to help prevent outbreaks. This includes efforts, such as the 2022 inspections and sampling in the Salinas Valley region, to shed light on potential routes of contamination during production, rather than conducting investigation(s) of fallow fields in the aftermath of an outbreak. Other important efforts underway include the FDA’s work with the produce industry to build and maintain a leafy greens data trust that would inform growers and other stakeholders, including the FDA, about potential food safety issues and the actions taken to address them.
The FDA also has been building capacity within and outside the agency to conduct effective root cause analysis, through training. Root cause analysis by industry is critical to determining sources and routes of contamination because industry is in the best position to identify food safety issues on the farm and to take action to address them to prevent contaminated produce from entering the marketplace.
The nation’s broad effort to prevent outbreaks of foodborne illness involving leafy greens also entails important work initiated by industry, academia and state regulators. The California LGMA has developed an Environmental Risk Assessment Tool and Western Growers has published an industry-developed guidance document to aid farms in conducting a root cause analysis. The California Agricultural Neighbors, established and convened by CDFA, is working to promulgate food safety best practices among farms.
Leafy greens require appropriate protection from human pathogens during growing, harvesting, packing and holding. The outbreaks of recent years and 2022 inspectional findings by CDFA, considered jointly, underscore the need for growers to continue to work toward compliance with the FDA’s Produce Safety Rule in order to protect the public health.
For consumer information on leafy greens food safety, visit the Centers for Disease Control and Prevention (CDC) web site.
[1] A traceback investigation is the method used to determine and document the production and distribution chain as well as the source(s) of a product that has been implicated in a foodborne illness investigation.
[2] FDA Center for Outbreak Response and Evaluation (CORE): Public Health Advisories from Investigations of Foodborne Illness Outbreaks. The “past five years” refers to 2018 to 2022.
[3] USDA: 2017 Census of Agriculture – Table 29
[4] The entities identified by the traceback investigations may be best characterized as “farm businesses.” The traceback investigations identified entities that in many cases owned or contracted for the use of more than one parcel of farmland. Additionally, all the farms (or entities) encompassed more than one field.
[5] The FDA and CDFA use three terms to classify inspectional outcomes: Official Action Indicated (OAI), Voluntary Action Indicated (VAI), and No Action Indicated (NAI). An “OAI” classification indicates significant violations and is accompanied by regulatory action. A “VAI” classification indicates lesser violation(s).
[6] “Preliminary Indication” suggests a sample may yield a final result that could indicate a public health threat. Analytical testing remains ongoing and final results have not yet been determined.
[7] CDFA inspected this farm on July 28, 2022. The inspectional outcome was NAI. The FDA collected the sample about two months following the inspection (on September 26, 2022).