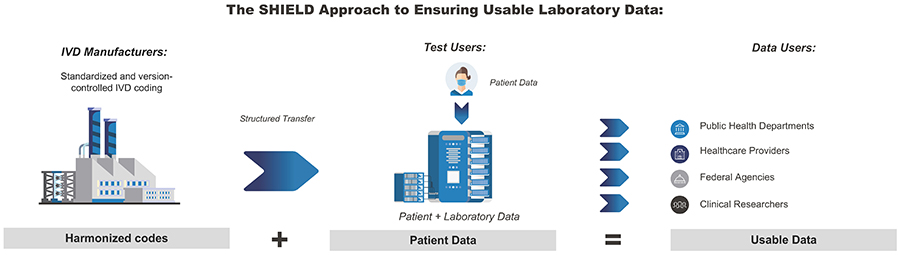
Systemic Harmonization and Interoperability Enhancement for Laboratory Data (SHIELD)
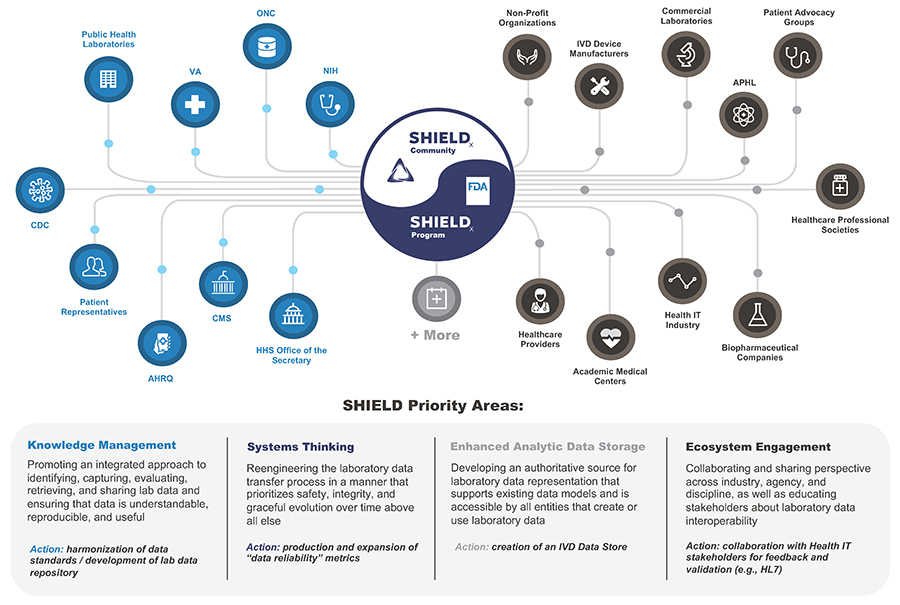
Since 2015, the FDA has partnered with federal agencies and key stakeholders from 70+ organizations (including federal, academia, and industry) to form and grow the Systemic Harmonization and Interoperability Enhancement for Laboratory Data (SHIELD) collaboration. SHIELD aims to bring together the brightest minds from across the laboratory data ecosystem to build, implement, and support a comprehensive solution that addresses clinical and semantic device interoperability of in vitro diagnostics (IVD) across the nation. This encompasses data as it flows throughout the entire laboratory data life cycle (that is, the exchange of data from standards organizations to IVD devices to laboratory workstations to Electronic Health Records [EHR]).
In 2023, key stakeholders established the SHIELD Collaborative Community. Members of the FDA SHIELD program team participate in this collaborative community.
Through the innovative harmonization of data standards such as SNOMED-CT (a U.S. government system used for the exchange of electronic clinical information) and LOINC (a database and universal standard for identifying medical laboratory observations), the FDA SHIELD program team is working to develop a platform that dramatically improves the quality and portability of laboratory data, which helps reduce patient safety risk. This work will be done in cooperation with leading laboratory data experts across the nation, prioritizing knowledge management, systems thinking, enhanced analytic data storage, and ecosystem engagement.
Challenges with Data Collection and Analysis
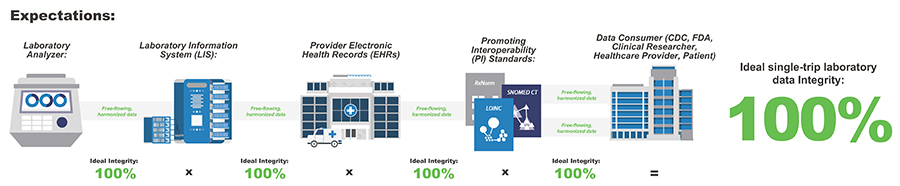
Clinical laboratory data – orders, results, and interpretations – are among the most important types of data for clinical care, public health, and the development of medical devices. Because of its widespread use, laboratory data has the potential to move freely between different systems.
Unfortunately, laboratory data is simple to transmit, but tremendously challenging to manage and evaluate due to non-standardized encoding practices used by IVD manufacturers, laboratories, and EHR vendors nationwide. This diversity in IVD test data may have an impact on patient safety and make it more difficult to analyze lab data on a national level.
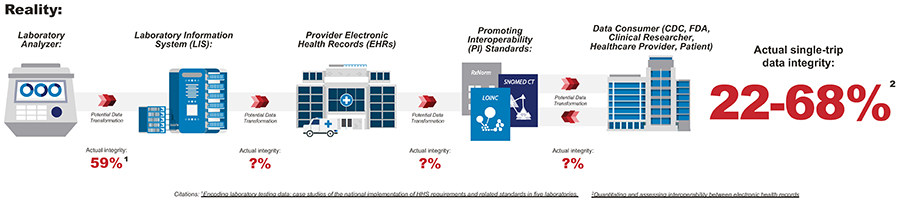
Researchers conducted an evaluation with five health care systems that found data maintained only 59% integrity as it moved from laboratory analyzer to laboratory information system. Similarly, the authors of a Journal of the American Medical Informatics Association (JAMIA) article estimated that the integrity of a single round-trip through the lab data lifecycle yielded 22-68% integrity.
Though testing methods and devices across laboratories throughout the country remain consistent, there are significant challenges that impede data collection and analysis and could adversely impact patients, providers, and organizations.
- There is no systematic application of codes by device manufacturers and data standards are not harmonized.
Impact: significant variation in organization, categorization, and storing of results - Existing standard interface specifications between IVDs and LISs are not implemented consistently across systems
Impact: there is room for manual translation and user-error - There is no hierarchy of concepts in the primary laboratory data standard
Impact: multiple available codes with varying levels of granularity for the same test - Not all relevant and pertinent data elements are codified or even included in the current interface implementations
Impact: valuable, useful information is not captured or codified
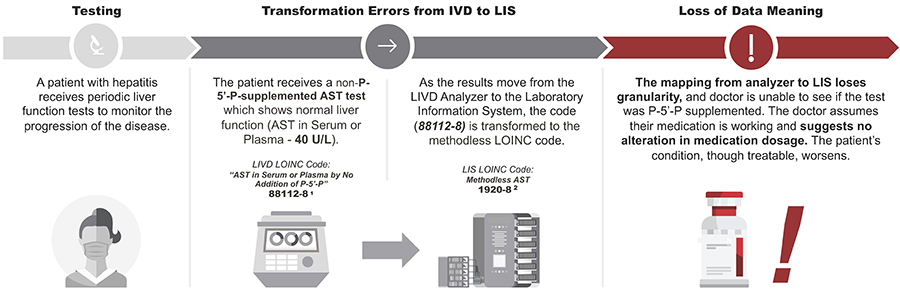
Example of Challenges
For patients with high levels of AST or Vitamin B6 deficiency, AST can be under-measured when the test method is not supplemented with P-5'-P.
Real world data (RWD), or using data collected as part of routine clinical care, is integral for product and policy decisions. However, there are often data siloes and incompatible health IT systems in health care that make data difficult to exchange and interpret.
Citations: https://loinc.org/88112-8/ 1, https://loinc.org/1920-8/ 2
Reference Articles: https://doi.org/10.1373/clinchem.2014.228247, https://www.linkedin.com/pulse/lab-result-interoperability-ast-andrea-pitkus-phd-mls-ascp-cm/
As the COVID-19 virus continued to spread and the "PCR test," or polymerase chain reaction test, became a familiar term, Congress and the public began to recognize the challenge of reporting test data in a timely and accurate manner. As symptoms changed and new variants of the virus were identified, the health technology community quickly discovered that existing health record systems and data representation processes were not agile enough to keep up. This lack of agility often manifested in a loss of COVID-19 data's accuracy or granularity.
Capturing usable diagnostic data from COVID-19 tests is of paramount importance to guide an evidence-based pandemic response at the local, state, and national levels. A lack of standards, interoperability, and connectivity by modern public health IT highways has led to fragmented, delayed, and incomplete testing data. Improvements in these areas will help strengthen the nation's health data infrastructure and public health readiness.
To address these issues, SHIELD offers uniform national coding standards for all IVDs, enabling the data reporting and analysis needed to continue to combat COVID-19. As a vital aspect of U.S. pandemic preparedness, SHIELD enables interoperable data capture and flow from all tests reported to public health authorities. Building the foundation for lab data interoperability using lessons learned from COVID-19 diagnostics will help prepare the FDA and the country for the next pandemic.
Using high-quality, standardized post-market data to support and enhance the review and authorization of novel diagnostic tests is another crucial requirement that our project addresses. By gathering and analyzing real-world data (RWD) and real-world evidence (RWE) from tests, developers and regulators will be able to both expedite the pre-market review of tests as well as assess their post-market, or real-world, performance. This will greatly improve the ability to evaluate the performance of tests as they are being used, and inform any necessary changes or modifications needed to improve the diagnostic accuracy of tests available to patients.
Through efforts such as the Diagnostics Evidence Accelerator, the importance of gathering RWD/RWE has been explored with numerous stakeholders. The immediate utility and power of this data will only be realized if the post-market data (RWD/RWE) gathered is harmonized and of high quality, exactly what the SHIELD collaborative seeks to accomplish.
To improve the quality, interoperability, and portability of IVD laboratory data within and between institutions and individuals, SHIELD is working hard to build a publicly accessible infrastructure. Analyzing diagnostic data from tests used during the pandemic will help SHIELD better understand the true accuracy and performance of different tests used in different settings and populations under real-world conditions. SHIELD is constantly learning about ways to improve the use, interpretation, and performance of tests–a process that relies on RWD/RWE and ultimately improves the medical products available to patients.
SHIELD Funded Program Portfolio
Awardee: Synensys LLC
Today, clinical laboratory safety and quality are managed across each level of the laboratory ecosystem; the device, individual laboratory, Laboratory Information System (LIS), the Electronic Health Record (EHR), hospitals, and information exchanges. However, the sum of the parts does not necessarily equal the whole–managing quality at the individual level alone is not sufficient to address safety and quality issues for the entire ecosystem. To help with this issue, the FDASHIELD program team is collaborating with team members from Synensys, LLC, a safety management system consulting firm, to assess, measure, document, and analyze the safety and quality of the entire laboratory data ecosystem.
Research in this project is not only focused on examining individual laboratory data adverse events that lead to preventable patient harm, but also system-level hazards that impact entire patient populations that rely on laboratory data. This project seeks to develop an accurate safety analysis of the current laboratory data ecosystem using a System Safety engineering approach, provide recommendations for a future system redesign to address system hazards by implementing new or redesigned control structures, and present a clear demonstration and justification of the urgency for system redesign to address critical safety hazards and risks to public health.
Awardee: College of American Pathologists (CAP)
While the Centers for Medicare and Medicaid Services (CMS) regulates clinical laboratories and requires that laboratories participate in proficiency testing for certain analytes, issues continue to exist with encoding laboratory test result data with standardized terminology necessary for interoperable data exchange and analytics. To help with this issue, the FDA SHIELD program team is collaborating with the CAP to design an ongoing laboratory data quality assurance and improvement program which will evaluate IVD test result coding and determine the consistency and accuracy of laboratory encoded data.
The scope of this work is to promote data quality and interoperability in laboratory encoded test result data. Two core objectives support this work: the development of a strategy to utilize laboratory data that has been encoded using industry coding standards (e.g., standard terminologies), and the design of a quality assurance program focused on improving the accuracy of laboratory test code set.
Awardee: Deloitte Consulting LLP
Clinical laboratory data is among the most important types of data for clinical care, public health, and the development of drugs and medical devices. Unfortunately, however, laboratory data is often encoded differently within and between institutions, meaning manual and error-prone techniques are required to determine equivalence in reporting systems.
To help improve the consistency and reliability of laboratory data encodings, the FDA SHIELD program team is collaborating with Deloitte Consulting to build a knowledge management solution that can be used by laboratories and public health agencies to promote data quality, data agility, and interoperability across the ecosystem. This solution will serve as a reference implementation of current HL7 Tinkar project efforts, which seeks to integrate, rather than replace, existing laboratory and non-laboratory terminology standards (e.g., SNOMED, LOINC) in a singular location for viewing, authoring, and editing.
Contact Us
For additional information, contact SHIELD-LabCodes@fda.hhs.gov.