Expanded Access (Compassionate Use) Submission Data
CDER, CBER and CDRH Expanded Access INDs and Protocols (2019-2023)
On this page you will find:
- The Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) expanded access submission receipt reports for INDs and Protocols from 2019-2023
- The Center for Devices and Radiological Health (CDRH) expanded access requests from 2019-2023
The reports are broken down to the following:
FY 2019 – 2023 (most recent 5 years) Graphs of Expanded Access Submissions
To view the graph select the appropriate link below.
For older graphs, see Expanded Access (Compassionate Use) Submission Data Archive: CDER and CBER and Expanded Access (Compassionate Use) Submission Data Archive: CDRH.
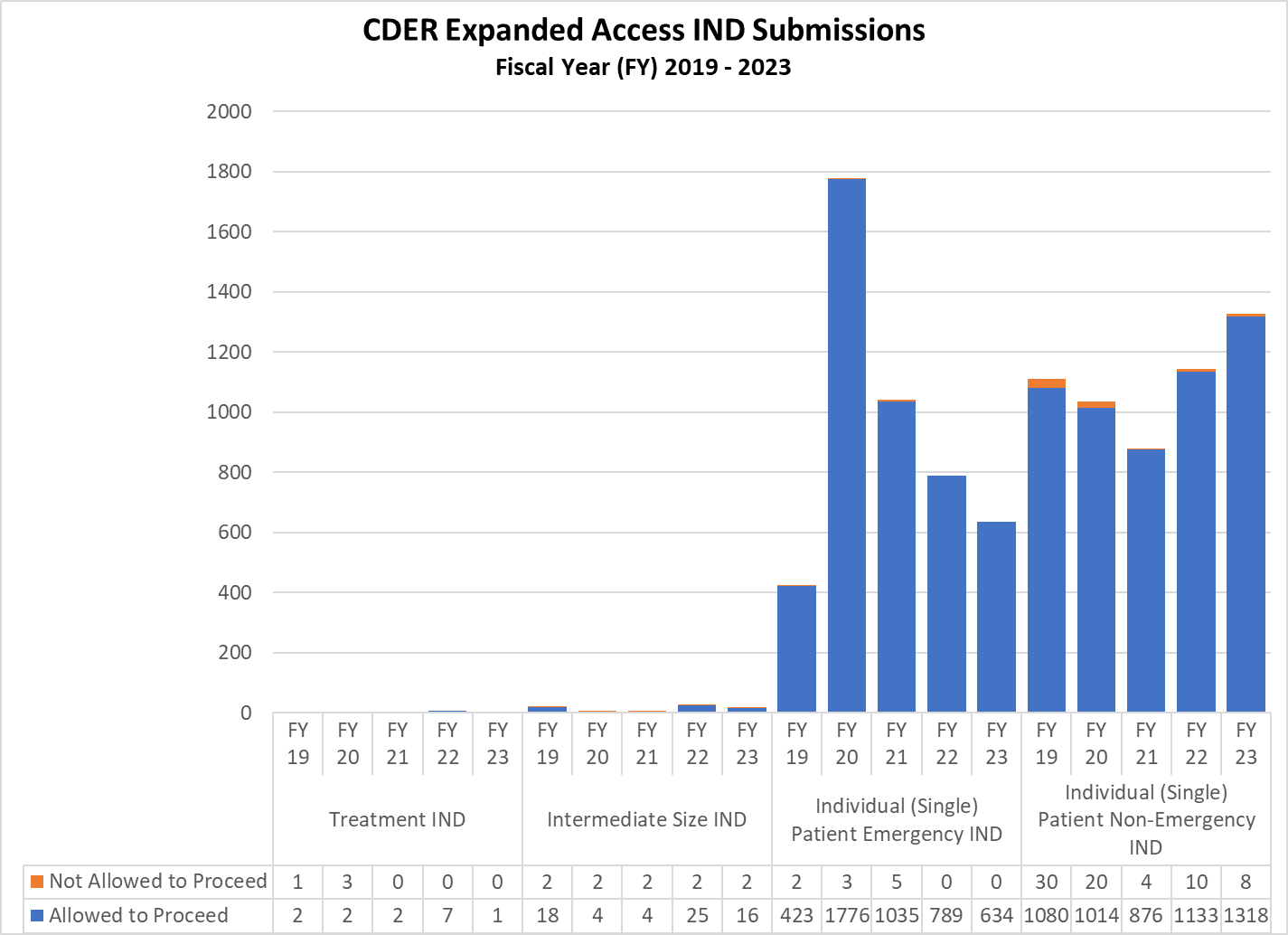
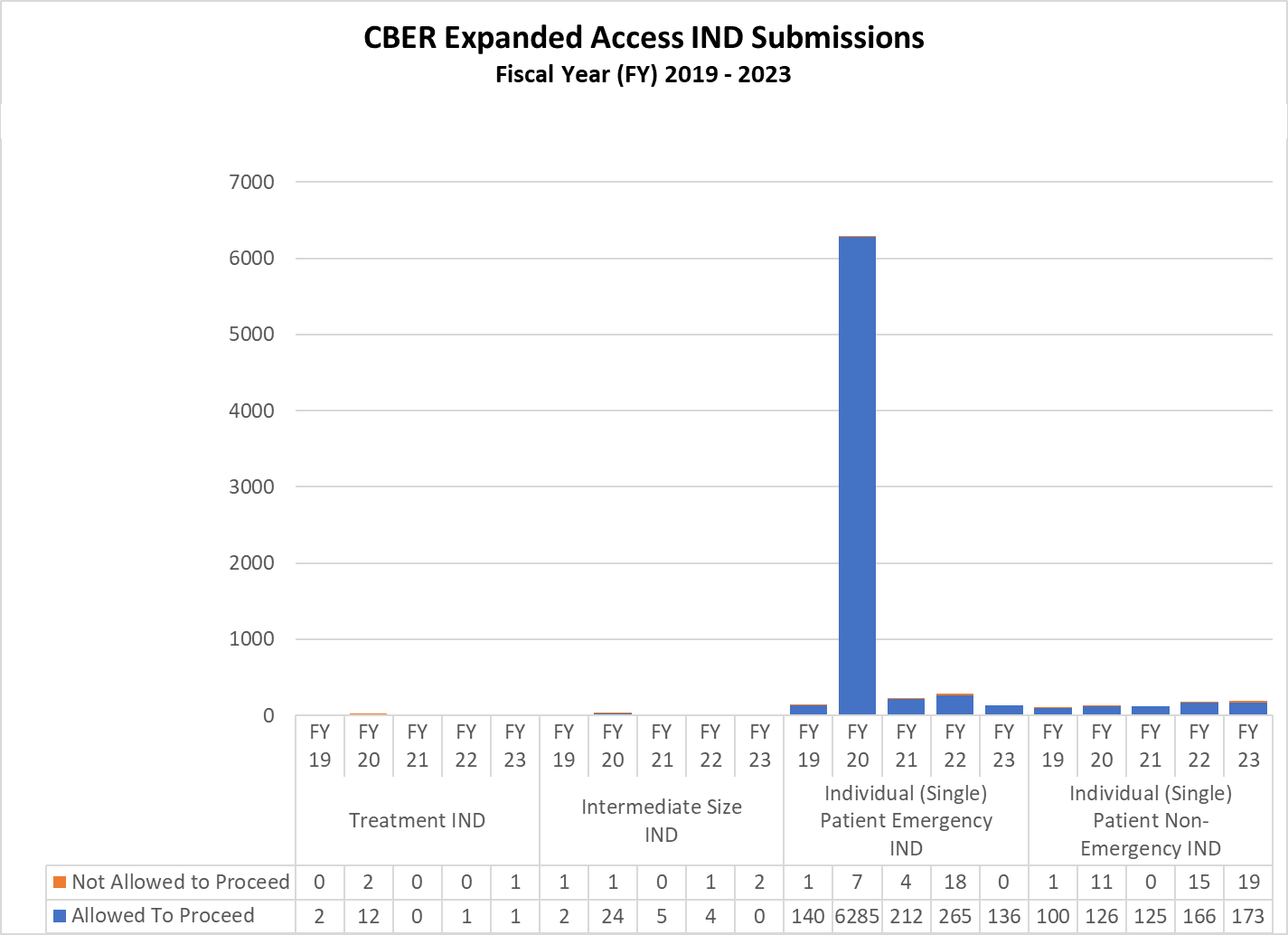
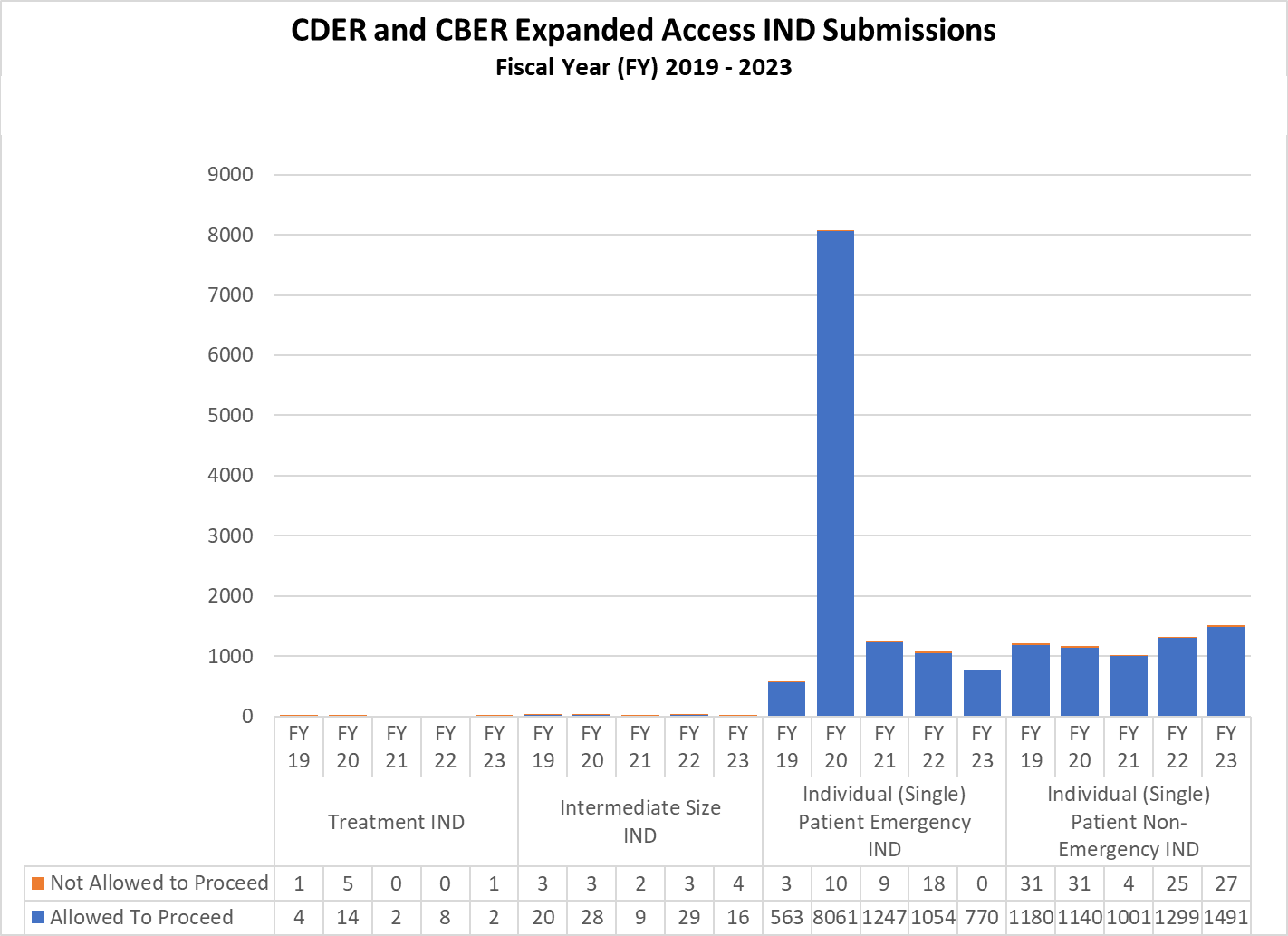
Expanded Access INDs for CDER and CBER (2019-2023)
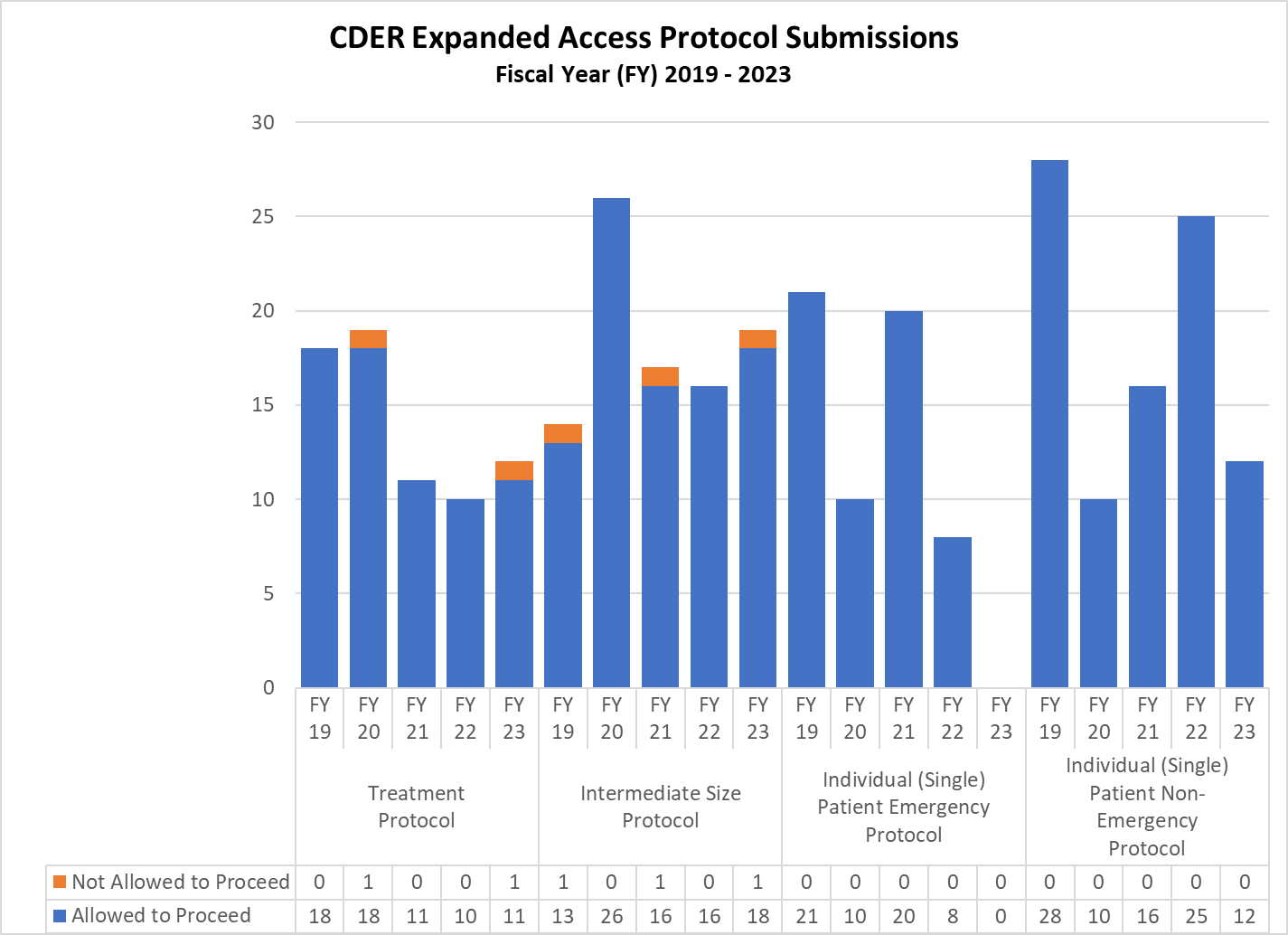
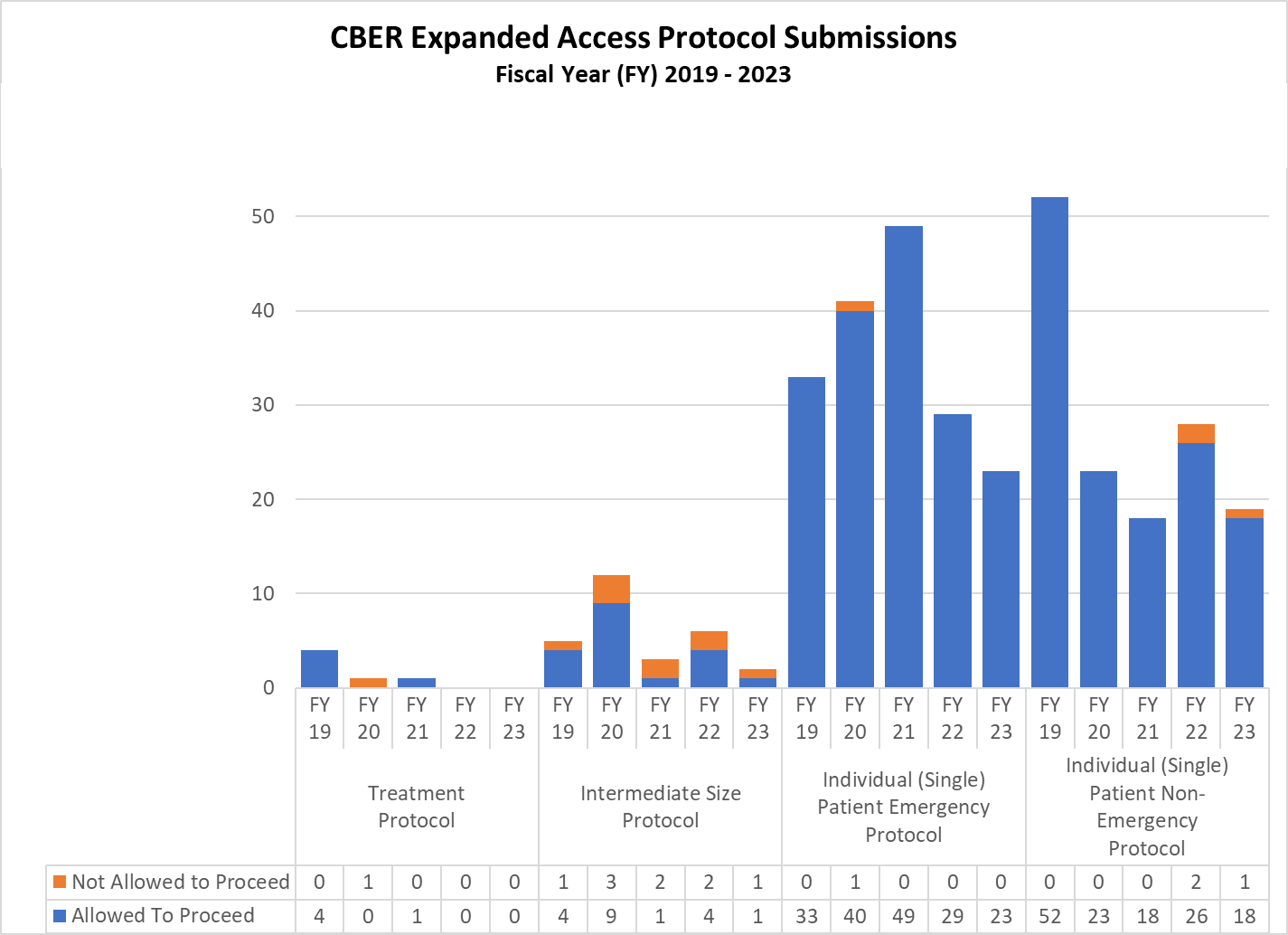
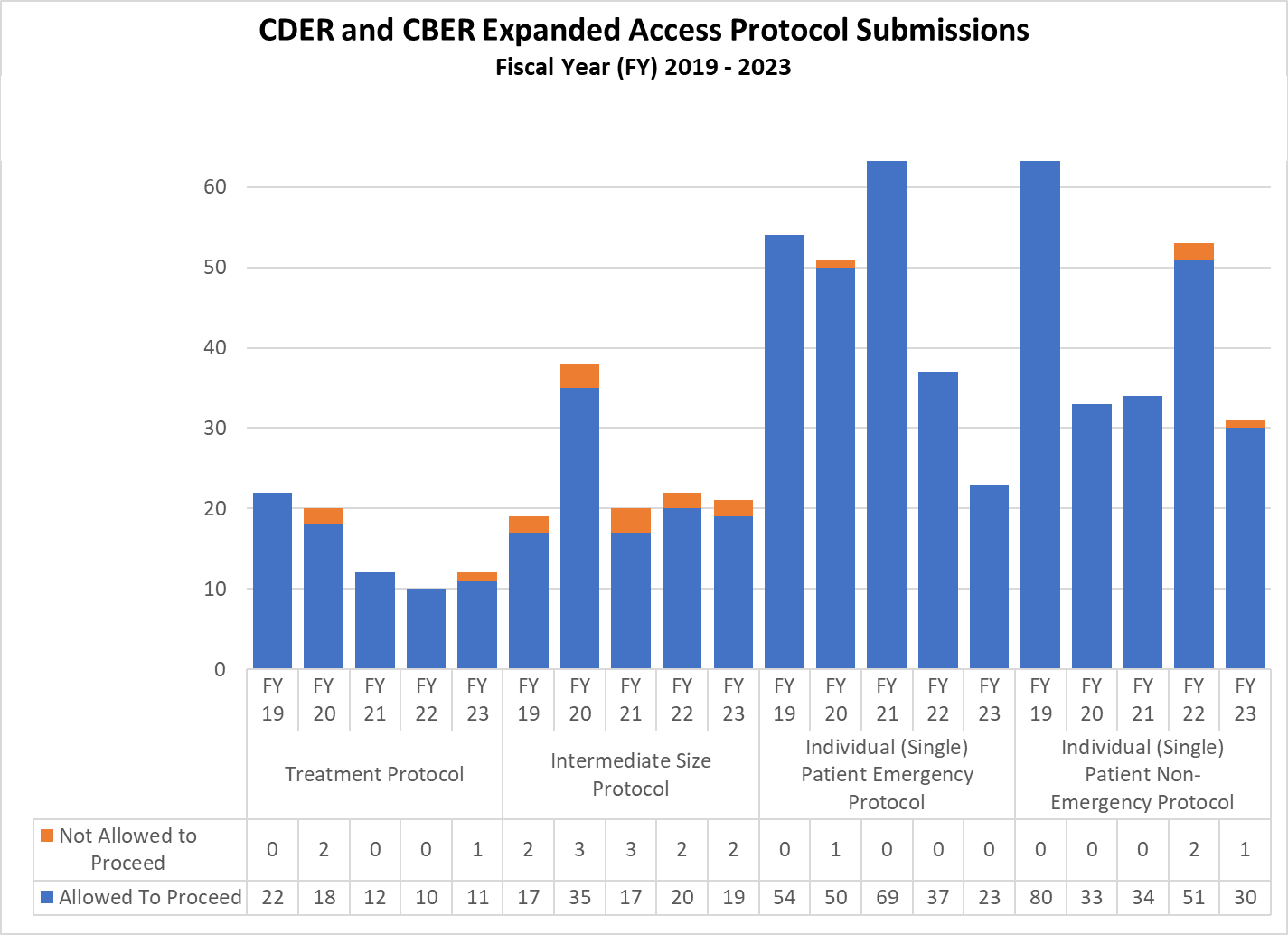
Expanded Access Protocols for CDER and CBER (2019-2023)
FY 2019 – 2023 Graphs of Expanded Access Submissions
CDER Expanded Access INDs and Protocol (2019-2023)
CBER Expanded Access INDs and Protocols (2019-2023)
Combined CDER and CBER Expanded Access Submissions and Protocols (2019-2023)
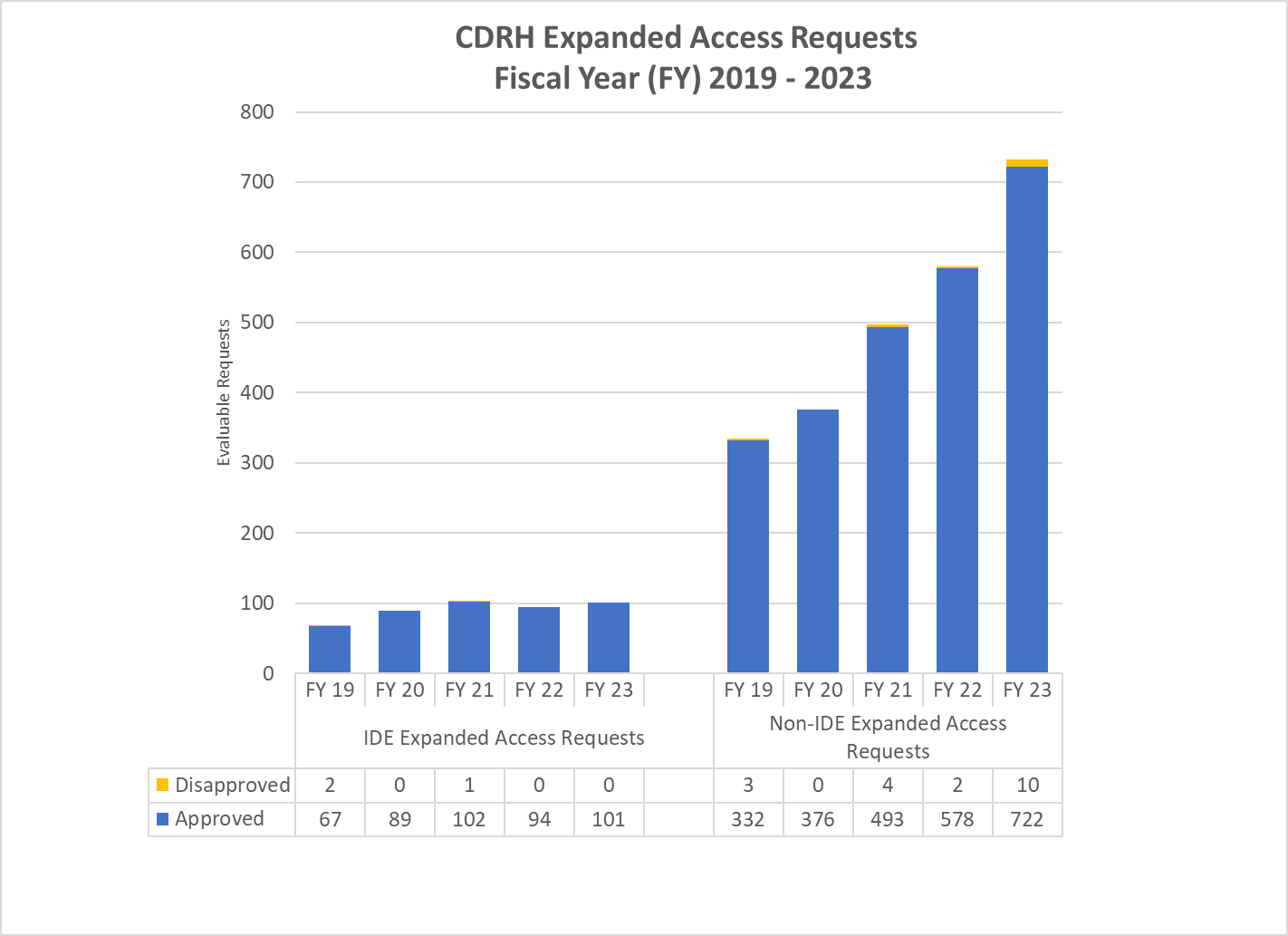
CDRH Expanded Access Requests (2019-2023)
FY 2019-2023 Graph of CDRH Expanded Access Requests
CDRH IDE Expanded Access Requests (2019-2023)1
| Expanded Access Totals | FY 2019 | FY 2020 | FY 2021 | FY 2022 | FY 2023 |
|---|---|---|---|---|---|
| Requests Received | 76 | 92 | 106 | 101 | 104 |
| Evaluable Requests2 | 69 | 89 | 103 | 94 | 101 |
| Approved | 67 | 89 | 102 | 94 | 101 |
| Percent Approved3 | 97.1% | 100% | 99.0% | 100.0% | 100.0% |
1 Expanded Access IDE requests in CDRH reflect compassionate use (single patient or small population) requests.
2 Evaluable requests are decisions in which a substantive review was performed on an expanded access request (individual patient or small group access) resulting in an approval, approval with conditions, or disapproval decision.
3 Based on approved requests to total number of evaluable requests.
CDRH Non-IDE Expanded Access Requests(2019-2023)1
| Expanded Access Totals | FY 2019 | FY 2020 | FY 2021 | FY 2022 | FY 2023 |
|---|---|---|---|---|---|
| Requests Received | 352 | 384 | 502 | 587 | 741 |
| Evaluable Requests2 | 335 | 372 | 497 | 580 | 732 |
| Approved | 332 | 372 | 493 | 578 | 722 |
| Percent Approved3 | 99.1% | 100% | 99.2% | 99.66% | 98.63% |
1 Expanded Access requests outside of an IDE are limited to single patient compassionate use requests.
2 Evaluable requests are requests in which a substantive review was performed on an original, non-IDE compassionate use request resulting in an approval/grant or disapproval/denial decision.
3 Based on approved/granted requests to total number of evaluable requests.