2023 FDA Science Forum
CarcSeq detection of a dose- and time-dependent induction of a cancer driver mutation in the mammary DNA of lorcaserin-treated rats
- Authors:
- Center:
-
Contributing OfficeNational Center for Toxicological Research
Abstract
Lorcaserin, a drug for weight management, is a selective agonist of the serotonin (5-hydroxytryptamine) 2C receptor. Although lorcaserin is a non-genotoxic rat carcinogen, FDA approval was granted in part based on dose extrapolation considerations. A post-marketing study, CAMELLIA-TIMI, designed to detect potential cardiovascular effects of lorcaserin therapy detected excess cancer risk in the lorcaserin treatment arm. Consequently, a study of lorcaserin-treated rats was conducted to elucidate the mechanism of lorcaserin-induced carcinogenesis and facilitate detection of other carcinogens operating through the same mechanism in the future.
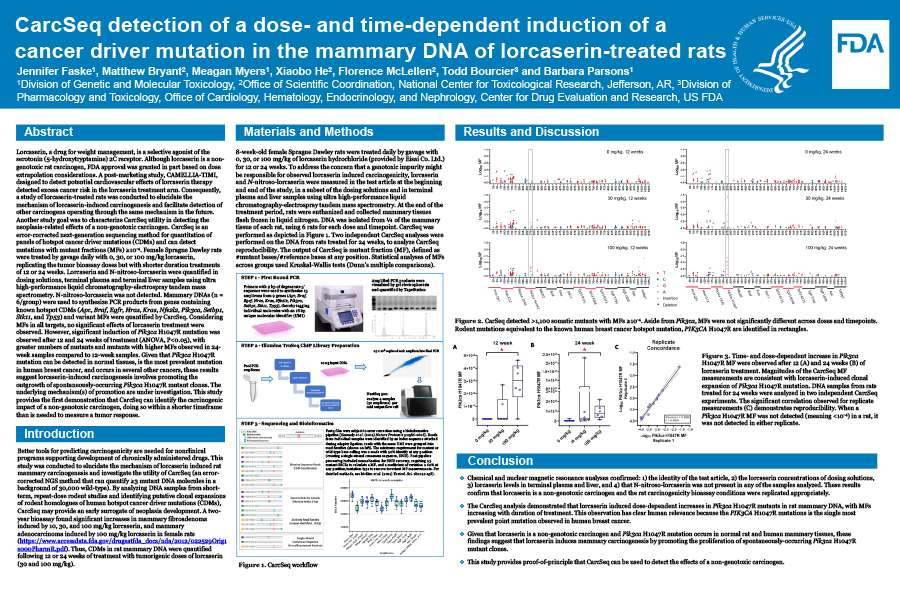
Another study goal was to characterize CarcSeq utility in detecting the neoplasia-related effects of a non-genotoxic carcinogen. CarcSeq is an error-corrected next-generation sequencing method for quantitation of panels of hotspot cancer driver mutations (CDMs) and can detect mutations with mutant fractions (MFs) ≥10-4. Female Sprague Dawley rats were treated by gavage daily with 0, 30, or 100 mg/kg lorcaserin, replicating the tumor bioassay doses but with shorter duration treatments of 12 or 24 weeks. Lorcaserin and N-nitroso-lorcaserin were quantified in dosing solutions, terminal plasma and terminal liver samples using ultra high-performance liquid chromatography-electrospray tandem mass spectrometry. N-nitroso-lorcaserin was not detected.
Mammary DNAs (n = 6/group) were used to synthesize PCR products from genes containing known hotspot CDMs (Apc, Braf, Egfr, Hras, Kras, Nfe2l2, Pik3ca, Setbp1, Stk11, and Tp53) and variant MFs were quantified by CarcSeq. Considering MFs in all targets, no significant effects of lorcaserin treatment were observed. However, significant induction of Pik3CA H1047R mutation was observed after 12 and 24 weeks of treatment (ANOVA, P<0.05), with greater numbers of mutants and mutants with higher MFs observed in 24-week samples compared to 12-week samples. Given that Pik3CA H1047R mutation can be detected in normal tissues, is the most prevalent mutation in human breast cancer, and occurs in several other cancers, these results suggest lorcaserin-induced carcinogenesis involves promoting the outgrowth of spontaneously-occurring Pik3CA H1047R mutant clones. The underlying mechanism(s) of promotion are under investigation.
This study provides the first demonstration that CarcSeq can identify the carcinogenic impact of a non-genotoxic carcinogen, doing so within a shorter timeframe than is needed to measure a tumor response.