2023 FDA Science Forum
FDA COVID-19 Critical Care Drug Monitoring Survey Portal - Ongoing Surveillance of Critical Drugs Related to COVID-19 Supply Disruptions
- Authors:
- Center:
-
Contributing OfficeCenter for Drug Evaluation and Research
Abstract
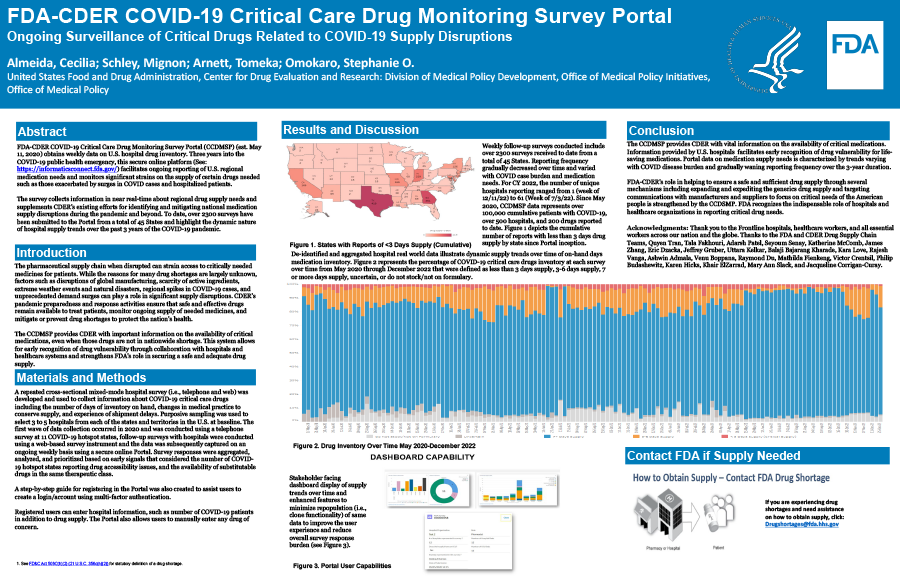
FDA COVID-19 Critical Care Drug Monitoring Survey Portal (CCDMSP), established May 11, 2020, obtains weekly data on U.S. hospital drug inventory by a voluntary survey based on a purposive sample. Three years into the COVID-19 public health emergency, this secure online platform (See: https://informaticsconnect.fda.gov/) facilitates ongoing reporting of U.S. regional medication needs and monitoring of significant strains on the supply of certain drugs needed such as those exacerbated by surges in COVID cases and hospitalized patients. While the reasons for drug shortages are multifactorial, issues such as disruptions of global manufacturing, scarcity of active ingredients, and drug demand surges can play a role. The CCDMSP collects repeated cross-sectional survey data and supplements FDA’s existing efforts for identifying and mitigating national medication supply disruptions during the pandemic and beyond.
This mixed-mode hospital survey (i.e., telephone and web) was developed and used to collect information about COVID-19 critical care drugs including the number of days of inventory on hand, changes in medical practice to conserve supply, and experience of shipment delays. Weekly follow-up surveys conducted include over 2300 submissions from a total of 45 States. For CY 2022, the number of unique hospitals reporting ranged from 1 (week of 12/11/22) to 61 (Week of 7/3/22). Since May 2020, over 100,000 cumulative patients with COVID-19, 500 hospitals, and 200 drugs are represented. De-identified and aggregated hospital real world data illustrate dynamic supply trends over time of on-hand days medication inventory.
The CCDMSP provides FDA with information on the availability of critical medications at point of care settings and provides additional supply chain visibility.