Office of Medical Device and Radiological Health Inspectorate (OMDRHI)
What We Do
- What should I expect during an inspection?
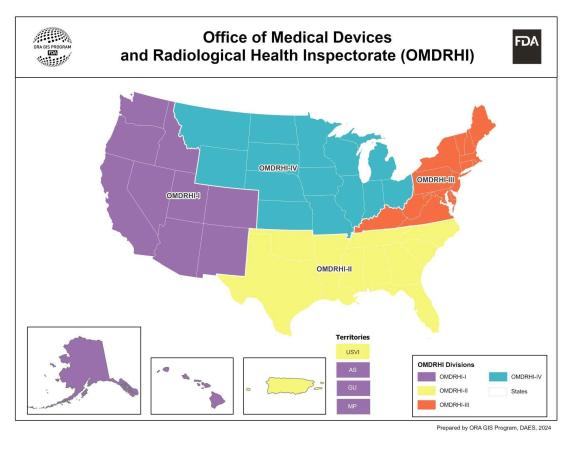
- New OII Boundary Map
- Communication Resources following an inspection:
- After an inspection – Obtaining the report
Field Management Directive 145 provides criteria and instructions for releasing one copy of an Establishment Inspection Report (EIR), to the firm’s top management official or designee at the inspected firm. EIRs will be transmitted via electronic means, when conditions in the FMD are met, from an ORADEVICES email (see below, per division) The EIR will come secured with a password issued in a separate email. If you have issues or concerns with your EIR, please reach out to the appropriate email address for resolution.- Division 1 West OII-DEVICES-West-FMD145@fda.hhs.gov
- Division 2 South OII-DEVICES-South-FMD145@fda.hhs.gov
- Division 3 Northeast OII-Devices-Northeast-FMD145@fda.hhs.gov
- Division 4 Midwest OII-Devices-Midwest-FMD145@fda.hhs.gov
- Foreign Branch CDRHforeigninspections@fda.hhs.gov
- Resources used by FDA investigators and inspectors in their daily activities
- 483 Response Information
How do I submit my FDA-483 Response following my inspection?
E-mail your inspection-related correspondence to the email address listed below. Please include your company’s FEI number, if known, in the subject of the email, and on the cover letter or documentation. Hard copy responses are discouraged, but if that is the only way you can send a response, please use the address listed below.
We prefer e-mail correspondence due to efficiency, fiscal responsibility, expedited service to stakeholders and environmental awareness. The Division will acknowledge receipt of your e-mail (size limit 100 megabytes) to the email box for your firm's geographic location.- Domestic:
- Division 1 West OII-DEVICES-West-Firm-Response@fda.hhs.gov
- Division 2 South OII-DEVICES-South-Firm-Response@fda.hhs.gov
- Division 3 Northeast OII-DEVICES-Northeast-Firm-Response@fda.hhs.gov
- Division 4 Midwest OII-DEVICES-Midwest-Firm-Response@fda.hhs.gov
- Foreign: cdrhforeigninspsections@fda.hhs.gov
Please be sure that any attachments are readily labeled and/or identified for ease of review to include the FEI number. Documentation should be submitted as a single pdf file, with bookmarks to easily identify table of contents, memos, attachments, etc. If a single pdf file exceeds the 100MB size limit, please submit multiple pdf files, with bookmarks, as appropriate. Please do not provide multiple folders that contain individual files as this will delay the processing of your response. There is no need to provide a back-up hard copy of any correspondence sent via email or provided in thumb drive or cd format. - Domestic:
Useful Links
- Final Rule: Quality Management System Regulation: Final Rule Amending the Quality System Regulation
- Quality Management System Regulation: Final Rule Amending the Quality System Regulation – Frequently Asked Questions
- Overview of Device Regulation
- Quality System Regulation/Medical Device Good Manufacturing Practices
- Device Advice: Comprehensive Regulatory Assistance
- Division of Industry and Consumer Education (DICE)
- CDRH Learn
- Unique Device Identification System (UDI System)
- MDSAP - Medical Device Single Audit Program
- MQSA - Mammography Quality Standards Act and Program
- A Day in the Life - Medical Device Program
- Our Work Our Voice - Hear OMDRHI employees describe their roles at FDA
- Consumer Complaints - How to Report Problems
- Import Program Resources
- Consumer Updates - Health Fraud
About the Office
The Office of Medical Device and Radiological Health Inspectorate (OMDRHI) is an office within the Medical Products Inspectorate (MPI) under the Office of Inspections and Investigations (OII). OMDRHI protects and promotes public health by conducting oversight, through inspections and investigations primarily of medical devices, radiological health products, and performs inspectional oversight of Mammography facilities. OMDRHI coordinates, directs, and conducts domestic and foreign inspections and investigations. OMDRHI manages medical device and radiological health recalls within our new Risk Mitigation and Response branch within our Division of Global Operations.
OMDRHI also provides direction and guidance to OII leadership and agency leaders relative to these areas that are regulated by Center for Devices and Radiological Health (CDRH). The office provides direction and counsel relative to medical device, mammography and radiological health field operations, in collaboration with CDRH, including response activities posed by potential threat to public health. In collaboration with CDRH, OMDRHI establishes inspection goals and annual workplan. OMDRHI supports the development of policy, develops guidance for inspections, and provides evidence to support compliance activities for medical device and radiological health firms and products.
Anne Reid directs the Office of Medical Devices and Radiological Health Inspectorate, and Rhonda Mecl is the deputy office director. OMDRHI has 6 divisions, including 4 domestic Division of Medical Device and Radiological Health Inspectorates (West, South, Northeast, and Midwest); Division of Medical Device and Radiological Health Global Operations (Operations Branch, Foreign Operations Branch, and Risk Mitigation and Response Branch); and Division of Mammography and Radiological Health Inspectorate (Mammography Operations Branch 1 and Mammography Operations Branch 2).
While we may have changed our name and a few elements of our structure, our Vision, Mission and dedication to our Quality Policy have not changed.
Vision
All patients, providers, and consumers are informed, protected, and have access to safe, reliable medical devices and radiological health products
Mission
Protect and enhance public health by minimizing risk and by supporting access to safe, effective and quality medical devices and radiological health products.
Quality Policy
OMDRHI will consistently provide products and services that meet or exceed the requirements and expectations of our customers. We will actively pursue improving quality through programs that enable each employee to do their job right the first time and every time.
Key Contacts
| Position | Name | |
|---|---|---|
| Office Director | Anne Reid | Anne.Reid@fda.hhs.gov |
| Deputy Office Director | Rhonda Mecl | Rhonda.Mecl@fda.hhs.gov |
Program Division Directors (PDD)
| Position | Name | |
|---|---|---|
| Northeast Division Director | Joseph Matrisciano | Joseph.Matrisciano@fda.hhs.gov |
| South Division Director | Blake Bevill | Blake.Bevill@fda.hhs.gov |
| West Division Director | Shari Shambaugh | Shari.Shambaugh@fda.hhs.gov |
| Midwest Division Director | Vacant | |
| Global Operations Division Director | Vacant | |
| Foreign Operations Branch Chief | Akbar Zaidi | Akbar.Zaidi@fda.hhs.gov |
| Mammography and Radiological Health Division Director | Vacant |