Companion Studies to Define the Distribution and Duration of Zika Virus in Non-Human Primates
Background | Project Description | Project Outcomes | Additional Reading
Performer: University of California, Davis
Project leader: Koen Van Rompay, DVM, PhD
Contract value: $144,890
Project dates: October 2016 – April 2017
Background
Prior to 2015, Zika virus outbreaks had occurred in areas of Africa, Southeast Asia, and the Pacific Islands. However, in May 2015, the Pan American Health Organization (PAHO) issued an alert (PDF, 199 KB) regarding the first confirmed Zika virus infection in Brazil. In 2015, outbreaks were occurring in many countries, including local transmission in parts of the continental United States.
Most people never know that they have been infected with Zika—in fact, four out of five patients with Zika virus infections are thought to have no symptoms at all. When symptoms do occur, the most common are fever, rash, joint pain, and conjunctivitis (red eyes). Even in those who develop symptoms, Zika illness is usually mild, with symptoms lasting from several days to a week.
Despite presenting as a mild illness in most patients, Zika has broader public health implications, including its potential impact on developing fetuses when pregnant women become infected. After careful review of existing evidence, Centers for Disease Control and Prevention (CDC) scientists determined that Zika virus is a cause of microcephaly,1 a condition in which a baby’s brain and head is smaller than expected, and other severe fetal brain defects.2 It can also cause Guillain-Barré syndrome, a disorder in which the immune system attacks the nervous system.
Zika can be transmitted by the bite of an infected Aedes species mosquito—found in large portions of the U.S.—and by sexual contact. FDA is taking steps to protect the blood supply to reduce the possibility of transfusion transmission, and recommending steps to reduce the risk of Zika virus transmission by human cell and tissue products, such as those intended for transplants.
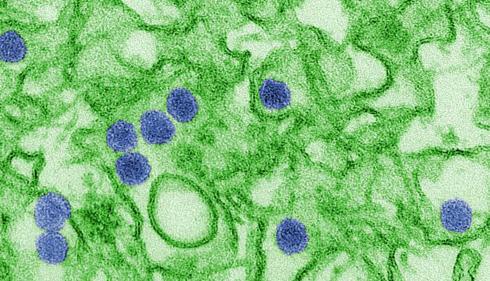
Image: Digitally colorized transmission electron microscopic (TEM) image of Zika virus (Credit: CDC/Cynthia Goldsmith)
Project Description
To learn more about how Zika affects the body, scientists are conducting nonclinical studies. In this FDA Medical Countermeasures Initiative (MCMi) regulatory science project, the University of California, Davis (UCD), will conduct laboratory studies to help inform FDA recommendations regarding potential transmission of Zika virus via organs and tissues.3 This study builds upon research conducted by the UC Davis California National Primate Research Center. ![]()
UCD will perform polymerase chain reaction (PCR) testing to screen for the presence of Zika virus RNA in samples taken from 38 different tissue types collected during a previous project, and test for infectious virus in positive samples. They will also perform histopathology and in situ hybridization, which uses labeled RNA as a probe, to locate and image Zika viral RNA in infected cells. This testing method enables researchers to better understand the pathogenesis of Zika virus.
Project Outcomes
As a result of this study, FDA will learn more about how Zika virus impacts a variety of tissues and organs in non-human primate models. This science will inform policy discussions regarding FDA guidance related to protecting blood, human cell and tissue product supplies, to better protect patients who receive these products from acquiring Zika virus.
Additional Reading
Companion Studies to Define the Distribution and Duration of Zika virus Infection in NHPs - final report (PDF, 5.6 MB - ARCHIVED)
Identification of viral reservoirs in Zika virus infected macaques to inform policies for human cells, tissues, and cellular and tissue-based products poster (PDF, 954 KB - ARCHIVED, presented on May 31, 2017 at the FDA Science Forum)
Coffey L, Keesler R, Pesavento P, et al. Intraamniotic Zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nature Communications. 2018 Jun 20;9:2414.
This project was funded through the MCMi Regulatory Science Extramural Research program.
Footnotes
1 In the April 13, 2016 report published in the New England Journal of Medicine, the CDC authors describe a rigorous weighing of evidence using established scientific criteria.
2 The finding that Zika virus infection can cause microcephaly and other severe fetal brain defects means that a woman who is infected with Zika during pregnancy has an increased risk of having a baby with these health problems. It does not mean, however, that all women who have Zika virus infection during pregnancy will have babies with problems. As has been seen during the current Zika outbreak, some infected women have delivered babies that appear to be healthy. More: Zika and pregnancy, from CDC
3 The California National Primate Research Center is part of the National Primate Research Centers Program at the National Institutes of Health.
